Except for the cystine crystals and a few others, the majority
of crystals found in the urinary sediment are of limited clinical
value. It is tempting to associate crystals with a risk of
urolithiasis, but the majority of patients with a crystalluria do
not have and will not develop kidney stones. Many benign
situations can provoke crystal formation.
In the majority of cases, the crystals found in urine are not
present in the freshly voided specimen. Alkalization and
refrigeration are promoters of crystals formation.
The interpretation of a persistent crystalluria must be done according to the clinic.
Drug crystals are sometimes found in urine. In most cases,
these findings are of little clinical value except, if the
sediment's picture indicates a possible renal obstruction.
Crystal casts are pathognomonic of this situation.
Some think that giving much time to the identification of unusual
crystals is not worthwhile.
Crystals related to urolithiasis are, except for cystine, usual
and easy to identify. Calcium is found in 80 to 95% of kidney
stones, mostly as oxalate or phosphate crystals. Many stones are
not homogeneous. Some have a nucleus of a different composition
from the surrounding matrix. The following table shows stones
composition listed by occurrence.
| Type | Occurence in % |
Calcium oxalate
|
70 |
Calcium phosphate
|
10 |
| Triple phosphates (Magnesium ammonium phosphate) (Struvite) | 5 to 10 |
| Uric acid | <5 |
| Cystine | 1 |
The elimination of these crystals can be made by gently heating the specimen at 37°C. To attain complete dissolution, it is preferable to heat the whole specimen. Once decanted, it is often impossible to dissolve the bulk in the small remaining volume.
It is possible to dissolve the obscuring crystals by adjusting the pH. Phosphates can be dissolved by adding a drop or two of 2% acetic acid. Amorphous urates can be dissolved by adding an alkali like a 2% ammonia solution. But heating is by far a preferred method. It is not wise to solve a urate problem by creating a phosphate precipitation.
It is impossible to dissolve the quantity of calcium, phosphate, and oxalate eliminated in a 24-hours urine specimen into 1 to 2 liters of water. It is therefore necessary to conclude that substances inhibiting the crystallization are present. Known inhibitors of urinary crystallization are pyrophosphate, citrate, magnesium, and certain macromolecules. The Tamm-Horsfall protein is believed to be an important calcium oxalate inhibitor. This role is thought to be due to the sialic acid residue of the protein. While the fully sialated protein is an inhibitor, the sialic residues lacking protein is a crystallization promoter.
Urine is a supersaturated solution of calcium, phosphate and oxalate in equilibria.
Crystal formation can be caused:
Some crystals are found exclusively in acid urine, others are found exclusively in alkaline urine.
Amorphous crystals are often identified on the basis of the urine pH. In an acid specimen, urates are reported, in an alkaline specimen, amorphous phospate are reported. This simplification should be used with care. Amorphous phosphates and triple phosphates are sometimes observed in slightly acid specimens. (pH 6,5)
| Alkaline pH | Acid pH |
| Amorphous phosphates | Amorphous urates |
| Triple phosphates | Uric acid |
| Ammonium biurates | Calcium oxalates |
| Calcium phosphates | |
| Calcium carbonates | Cystine |
Specific clinical conditions that explain urolithiase formation can also explain a persistent crystalluria.
An increased urinary calcium elimination can result in a crystalluria, mostly as calcium oxalates. The superior limit for the calciuria is 75 mmol/d under a 250 mmol/d diet.
Hypercalciuria can be caused by:
The calcium oxalate is probably the crystal that one meets the most frequently in a urinary sediment. In the majority of cases, the presence of these crystals is without any clinical meaning. According to Conyers, only 10 to 15% of the urinary oxalate is directly related to the diet. The majority of the urinary oxalates is produced by the metabolism (glyoxilic acid cycle). It seems that even light hyperoxaluria is, after the decreased urinary volume, the most significant factor in the recurrent calcium oxalate urolithiasis.
In some cases, the crystallization of the calcium oxalate is massive and catastrophic. A typical example of the oxalate clinical catastrophe is the cases of ethylene glycol poisoning. In this situation, one can find oxalate crystals in the patient's tissues. The toxicity syndrome affects organs like the liver, the kidney, and the brain and is accompanied by a metabolic acidosis. Naturally, the oxalate crystalluria is massive, and is predominated by the ovoid crystals (Whewellite) forming microlithes.
Calcium oxalate casts are highly significant. These imply that the oxalate crystals were already formed when the urine was at it's maximum dilution.
Conyers has reported other substances that can lead to oxalosis. Some of these substances are use as glucose substitutes in parental alimentation.
Other causes of hyperoxaluria are:
Fatty acids are competing with oxalate for the intestinal calcium. In fat malabsorbtion, the increase of unabsorbed fatty acids mobilizes the calcium leaving the oxalate, free to be absorbed. Intestinal calcium limits the absorption of oxalate.
An increased elimination of urate is most frequently caused by a high purine diet. Overproduction, can also be a cause of hyperuricosuria. With a urinary pH greater than 5,5, amorphous urates will be the major crystal form. Below 5.5, uric acid crystals are observed.
It is not rare to observe calcium oxalate crystals with amorphous urate in the same urinary sediment. Urate crystals seem to have an enhancer effect on the calcium oxalate crystal formation. A possible explanation is that urates and oxalates are competing for the litho-inhibitor macromolecules.
The chelating effect of citrate is known to reduce the saturation in calcium salt. Also, the soluble chelating calcium complex seems to have an inhibiting effect on crystal formation. One can therefore expect an increase in crystals formation, and even urolithiasis in conditions leading to hypocitraturia.
Hypocitraturia is seen in conditions like in:
Many bacteria infecting the urinary tract reduce the citrate concentration.
5% of the hypocitraturia are of unknown causes.
Approximately 66 to 75% of the uric acid is eliminated by the urine. The quantity to eliminate depends mostly on the diet (meat). In increased uricosuria, values > 4,5 mmol/d are observed.
Uric acid crystals are formed when the urinary pH is <5,5 since the pK of uric acid is 5,5.
Uric acid crystalluria is mainly due to a poor dilution volume at an acid pH, or due to an overproduction. In the majority of cases, this finding is of little clinical value and represents a pinpoint situation.
Some conditions, like chronic diarrhea, can be responsible for uric acid urolithiasis. Many of these patients will also have calcium stones.
Uric acid stones are seen in cases of gout, myeloproliferative syndrome, glycogenosis and neoplasms.
Cystine crystals are found in urine of almost exclusively patients with a genetic disease giving an impairment with the tubular reabsorption of the basic aminoacid: lysine, arginine, ornithine and cystine. This disease is called cystinuria. For a few patients with cystinuria, stones will develop. The urolithiasis is highly dependent of the urinary pH and water intake. Cystine is less soluble at a pH lower than 5,0 (saturation 300 mg/l); saturation is of 500 mg/l at a pH of 7,4.
Infection with urea splitting bacteria (ex: proteus species) leads to a production of ammonia an alkalinization of the urine. The produced ammonia generates magnesium ammonium phosphate crystals, also called triple phosphates. The mineralogical name of triple phosphate is Struvite. Triple phosphates are usually found with amorphous phosphates, owing to their low solubility at alkaline pH.
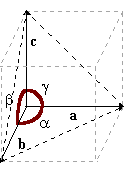
Solid substances are divided in two large groups, amorphous substances and crystalline substances. Crystals have defined geometrical shapes while amorphous substances have not. More, crystals have a precise melting point, while amorphous substances have a melting point that spreads over an interval of temperature. In crystallography, one speaks of planes, axes, and angles to describe their shape. Crystals, while preserving their primary shape, have a very variable size, but the ratios and angles between the faces and between the sides, are constant.
 A characteristic of crystals is that
their form is predictable from the elementary structure. There
are 230 possible geometrical forms that are grouped in 32
classes, based upon the arrangement of elements of symmetry.
These elements of symmetry are axes of symmetry, planes of
symmetry, the center of symmetry. These 32 classes are further
regrouped in 6 crystalline systems. Crystallographic constants of
these crystalline systems are described by a system of
coordinate, 3 axes (a, b, c), and by the angles formed by these
axes between them (a, b, g).
A characteristic of crystals is that
their form is predictable from the elementary structure. There
are 230 possible geometrical forms that are grouped in 32
classes, based upon the arrangement of elements of symmetry.
These elements of symmetry are axes of symmetry, planes of
symmetry, the center of symmetry. These 32 classes are further
regrouped in 6 crystalline systems. Crystallographic constants of
these crystalline systems are described by a system of
coordinate, 3 axes (a, b, c), and by the angles formed by these
axes between them (a, b, g).
System |
axes |
Bases |
Examples |
| Cubic | a = b = c a = b = g = 90° |
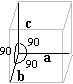 |
Apatite |
| Tetragonal | a = b <> c a = b = g = 90° |
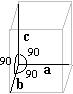 |
Calcium oxalate 2(H2O) « Weddellite » |
| Hexagonal | a = b <> c a = 120° b = g = 90° |
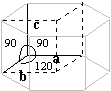 |
Cystine Tricalcium phosphate Apatite |
| Orthorombic | a = b = c a = b = g <> 90° |
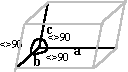 |
Triple phosphate Uric acid |
| Monoclinic | a <> b <>* c a = b= 90° et g <> 90° |
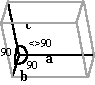 |
Calcium oxalate (H2O) « Whewellite » Calcium hydrogen phosphate Sodium acid urate |
| Triclinic | a <> b <> c a <> b <> g <> 90° |
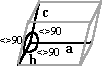 |
Sodium acid urate |
Crystals of the cubic system are said to be isotropic, since these have the same properties in all direction. One of these properties is the refractive index. Crystals of the other systems are said to be anisotropic, having two (birefringent) or even three refractive indexes. Anisotropic crystals subdivide into two groups: uniaxial (two refractive indexes) and biaxial (three refractive indexes). Crystals of the tetragonal system and the hexagonal system are uniaxial while the orthorombic, monoclinic and the triclinic are biaxial.
Some anisotropic crystals form interference patterns when viewed under polarized light. Uric acid are polychromatic, and cholesterol ester in a liquid crystal state generates a maltese cross pattern.
Birefringent is a crystal property
The following table shows the birefringent behavior of some crystals found in urine.
| None to light | Moderate | Strong |
| Amorphous phosphates | Cystine | Uric acid |
| Triple phosphates | Calcium oxalates 2(H2O) Weddelite |
Urates |
| Tricalcium phosphates | Calcium oxalates (H2O) Whewellite |
|
| Leucine |
Urine is a complex medium which influences the crystallization process. The same substance can crystallize into different shapes depending on the urine composition. Crystals found in urine are often truncated and eroded. Spherical crystals are frequent.
Slow crystallization tends to give larger crystals.