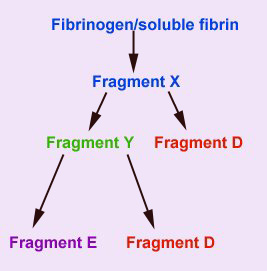
This assay detects the presence of circulating fragments (FDPs) of fibrinogen and soluble (non-crosslinked) fibrin that are produced by the action of plasmin on these substrates. Plasmin acts on these 2 substrates similarly, producing an initial cleavage product called fragment X. Plasmin then acts on fragment X, cleaving it into a transient fragment Y, and fragment D. Further cleavage of fragment Y, produces the terminal fibrin(ogen) degradation products, fragment D and E. Thus from one molecule of fibrinogen or soluble fibrin, 2 terminal FDP fragments D and one terminal fragment E is produced (as shown below).
 There are assays for FDPs in both serum and plasma. Serum FDP assays use polyclonal antibodies that cross-react with intact fibrinogen (that has not been lysed by plasmin), necessitating its removal. This is accomplished by the use of specialized collection tubes containing Botrox atrox venom (Reptilase)and inhibitors of fibrinolysis (aprotonin or soyabean trypsin inhibitor). All serum FDP assays use latex beads that are coated with antibody raised against human fibrin(ogen) degradation products, usually fragments D and E. The antibodies cross-react well enough with the fibrin(ogen) fragments of animals to make the test useful for veterinary patients.
There are assays for FDPs in both serum and plasma. Serum FDP assays use polyclonal antibodies that cross-react with intact fibrinogen (that has not been lysed by plasmin), necessitating its removal. This is accomplished by the use of specialized collection tubes containing Botrox atrox venom (Reptilase)and inhibitors of fibrinolysis (aprotonin or soyabean trypsin inhibitor). All serum FDP assays use latex beads that are coated with antibody raised against human fibrin(ogen) degradation products, usually fragments D and E. The antibodies cross-react well enough with the fibrin(ogen) fragments of animals to make the test useful for veterinary patients.  Two mls (as little as 0.5 mls can be used if the patient is very small) of blood from the patient is put immediately into one of the tubes provided with the test kit (see image on the right). Serum from the tube is diluted 1:5 and 1:20. A small volume of each dilution is mixed on a plate with an equal volume of the antibody-coated latex beads. Positive and negative control sera are assayed concurrently. Agglutination of the beads at the 1:5 dilution indicates an FDP concentration of 10-40 µg/ml, whereas agglutination at both 1:5 and 1:20 dilutions indicates an FDP concentration >40 µg/ml. Either positive result is abnormal and indicates a higher than normal rate of degradation of fibrinogen and/or fibrin by plasmin or decreased clearance of these products. Two mls (as little as 0.5 mls can be used if the patient is very small) of blood from the patient is put immediately into one of the tubes provided with the test kit (see image on the right). Serum from the tube is diluted 1:5 and 1:20. A small volume of each dilution is mixed on a plate with an equal volume of the antibody-coated latex beads. Positive and negative control sera are assayed concurrently. Agglutination of the beads at the 1:5 dilution indicates an FDP concentration of 10-40 µg/ml, whereas agglutination at both 1:5 and 1:20 dilutions indicates an FDP concentration >40 µg/ml. Either positive result is abnormal and indicates a higher than normal rate of degradation of fibrinogen and/or fibrin by plasmin or decreased clearance of these products.
There are newer latex-agglutination kits that are based on monoclonal antibodies that do not cross-react with intact fibrinogen and can thus be used on citrated plasma samples. This is advantageous compared to serum FDP assays, as a specialized collection tube for FDP assay is not required and a single citrated blood sample can be used for all coagulation tests. This is the procedure performed by the Clinical Pathology laboratory at Cornell University and it has only been validated for the dog. The plasma FDP assay is performed similarly to the serum FDP assay, except dilutions of 1:2 and 1:8 are made, with results being reported as < 5 µg/ml, 5-20 µg/ml and > 20 µg/ml. Results > 5 µg/ml are abnormal. |
| The FDP assay is used with the other coagulation tests to characterize
bleeding disorders more completely. The results should never be interpreted
alone, without evaluation of clinical signs and results of other coagulation
tests. Any cause of pathologic intravascular coagulation (the most common
of which is DIC), thrombosis, or severe internal hemorrhage (see below)
can produce FDP's. In addition, any condition causing decreased clearance
of FDPs by the liver and monocyte-macrophage system, e.g. severe liver
disease, can also result in increased FDP values. A negative FDP result
does not rule out these processes.
Note that coagulation can also occur in extravascular tissue spaces
and high FDPs have been associated with fibrin(ogen)olysis occurring
in protein-rich or hemorrhagic effusions into body cavities. Indeed,
horses with colic due to gastrointestinal disorders have higher FDPs
in blood and peritoneal fluid than healthy control horses. Although
the high FDPs in the blood of these colic horses are compatible with
DIC (which is often subclinical in horses and results in thrombosis
rather than hemorrhage), the FDP values in their peritoneal fluid were
higher than in blood, suggesting extravascular production of the FDPs
(probably secondary to inflammation-induced tissue factor expression
and fibrinolysis in the peritoneal cavity). This could have partly contributed
to the high blood FDPs.
The serum FDP assay is of little use when evaluating horses for coagulopathies
because of the high frequency of false positive results. Sera from most
horses, whether healthy or ill, give positive tests with this assay.
A single plasma assay has been evaluated and does not appear to crossreact
with equine FDPs (all these latex agglutination assays use antibodies
raised against human FDPs).

|
|