Ed Friedlander, M.D., Pathologist
scalpel_blade@yahoo.com


Cyberfriends: The help you're looking for is probably here.
Welcome to Ed's Pathology Notes, placed here originally for the convenience of medical students at my school. You need to check the accuracy of any information, from any source, against other credible sources. I cannot diagnose or treat over the web, I cannot comment on the health care you have already received, and these notes cannot substitute for your own doctor's care. I am good at helping people find resources and answers. If you need me, send me an E-mail at scalpel_blade@yahoo.com Your confidentiality is completely respected.
 DoctorGeorge.com is a larger, full-time service.
There is also a fee site at myphysicians.com,
and another at www.afraidtoask.com.
DoctorGeorge.com is a larger, full-time service.
There is also a fee site at myphysicians.com,
and another at www.afraidtoask.com.
Translate this page automatically
 |
With one of four large boxes of "Pathguy" replies. |
 I'm still doing my best to answer
everybody.
Sometimes I get backlogged,
sometimes my E-mail crashes, and sometimes my
literature search software crashes. If you've not heard
from me in a week, post me again. I send my most
challenging questions to the medical student pathology
interest group, minus the name, but with your E-mail
where you can receive a reply.
I'm still doing my best to answer
everybody.
Sometimes I get backlogged,
sometimes my E-mail crashes, and sometimes my
literature search software crashes. If you've not heard
from me in a week, post me again. I send my most
challenging questions to the medical student pathology
interest group, minus the name, but with your E-mail
where you can receive a reply.
Numbers in {curly braces} are from the magnificent Slice of Life videodisk. No medical student should be without access to this wonderful resource. Someday you may be able to access these pictures directly from this page.
Also:
Medmark Pathology -- massive listing of pathology sites
Freely have you received, freely give. -- Matthew 10:8. My
site receives an enormous amount of traffic, and I'm
handling about 200 requests for information weekly, all
as a public service.
Pathology's modern founder,
Rudolf
Virchow M.D., left a legacy
of realism and social conscience for the discipline. I am
a mainstream Christian, a man of science, and a proponent of
common sense and common kindness. I am an outspoken enemy
of all the make-believe and bunk that interfere with
peoples' health, reasonable freedom, and happiness. I
talk and write straight, and without apology.
Throughout these notes, I am speaking only
for myself, and not for any employer, organization,
or associate.
Special thanks to my friend and colleague,
Charles Wheeler M.D.,
pathologist and former Kansas City mayor. Thanks also
to the real Patch
Adams M.D., who wrote me encouragement when we were both
beginning our unusual medical careers.
If you're a private individual who's
enjoyed this site, and want to say, "Thank you, Ed!", then
what I'd like best is a contribution to the Episcopalian home for
abandoned, neglected, and abused kids in Nevada:
My home page
Especially if you're looking for
information on a disease with a name
that you know, here are a couple of
great places for you to go right now
and use Medline, which will
allow you to find every relevant
current scientific publication.
You owe it to yourself to learn to
use this invaluable internet resource.
Not only will you find some information
immediately, but you'll have references
to journal articles that you can obtain
by interlibrary loan, plus the names of
the world's foremost experts and their
institutions.
Alternative (complementary) medicine has made real progress since my
generally-unfavorable 1983 review linked below. If you are
interested in complementary medicine, then I would urge you
to visit my new
Alternative Medicine page.
If you are looking for something on complementary
medicine, please go first to
the American
Association of Naturopathic Physicians.
And for your enjoyment... here are some of my old pathology
exams
for medical school undergraduates.
I cannot examine every claim that my correspondents
share with me. Sometimes the independent thinkers
prove to be correct, and paradigms shift as a result.
You also know that extraordinary claims require
extraordinary evidence. When a discovery proves to
square with the observable world, scientists make
reputations by confirming it, and corporations
are soon making profits from it. When a
decades-old claim by a "persecuted genius"
finds no acceptance from mainstream science,
it probably failed some basic experimental tests designed
to eliminate self-deception. If you ask me about
something like this, I will simply invite you to
do some tests yourself, perhaps as a high-school
science project. Who knows? Perhaps
it'll be you who makes the next great discovery!
Our world is full of people who have found peace, fulfillment, and friendship
by suspending their own reasoning and
simply accepting a single authority that seems wise and good.
I've learned that they leave the movements when, and only when, they
discover they have been maliciously deceived.
In the meantime, nothing that I can say or do will
convince such people that I am a decent human being. I no longer
answer my crank mail.
This site is my hobby, and I presently have no sponsor.
This page was last updated February 6, 2006.
During the ten years my site has been online, it's proved to be
one of the most popular of all internet sites for undergraduate
physician and allied-health education. It is so well-known
that I'm not worried about borrowers.
I never refuse requests from colleagues for permission to
adapt or duplicate it for their own courses... and many do.
So, fellow-teachers,
help yourselves. Don't sell it for a profit, don't use it for a bad purpose,
and at some time in your course, mention me as author and KCUMB as my institution. Drop me a note about
your successes. And special
thanks to everyone who's helped and encouraged me, and especially the
people at KCUMB
for making it possible, and my teaching assistants over the years.
Whatever you're looking for on the web, I hope you find it,
here or elsewhere. Health and friendship!
QUIZBANK Endocrine (It's impossible to separate pituitary, adrenal, thyroid, parathyroid, etc. Look at it all
now.)
 I am presently adding clickable links to
images in these notes. Let me know about good online
sources in addition to these:
I am presently adding clickable links to
images in these notes. Let me know about good online
sources in addition to these:
Pathology Education Instructional Resource -- U. of Alabama; includes a digital library
Houston Pathology -- loads of great pictures for student doctors
Pathopic -- Swiss site; great resource for the truly hard-core
Syracuse -- pathology cases
Walter Reed -- surgical cases
Alabama's Interactive Pathology Lab
"Companion to Big Robbins" -- very little here yet
Alberta
Pathology Images --hard-core!
Cornell
Image Collection -- great site
Bristol Biomedical
Image Archive
EMBBS Clinical
Photo Library
Chilean Image Bank -- General Pathology -- en Español
Chilean Image Bank -- Systemic Pathology -- en Español
Connecticut
Virtual Pathology Museum
Australian
Interactive Pathology Museum
Semmelweis U.,
Budapest -- enormous pathology photo collection
Iowa Skin
Pathology
Loyola
Dermatology
History of Medicine -- National Library of Medicine
KU
Pathology Home
Page -- friends of mine
The Medical Algorithms Project -- not so much pathology, but worth a visit
National Museum of Health & Medicine -- Armed Forces Institute of Pathology
Telmeds -- brilliant site by the medical students of Panama (Spanish language)
U of
Iowa Dermatology Images
U Wash
Cytogenetics Image Gallery
Urbana
Atlas of Pathology -- great site
Visible
Human Project at NLM
WebPath:
Internet Pathology
Laboratory -- great siteEd Lulo's Pathology Gallery
Bryan Lee's Pathology Museum
Dino Laporte: Pathology Museum
Tom Demark: Pathology Museum
Dan Hammoudi's Site
Claude Roofian's Site
Pathology Handout -- Korean student-generated site; I am pleased to permit their use of my cartoons
Estimating the Time of Death -- computer program right on a webpage
Pathology Field Guide -- recognizing anatomic lesions, no pictures
St.
Jude's Ranch for Children
I've spent time there and they are good. Write "Thanks
Ed" on your check.
PO Box 60100
Boulder City, NV 89006--0100
More of my notes
My medical students
Clinical
Queries -- PubMed from the National Institutes of Health.
Take your questions here first.
HealthWorld
Yahoo! Medline lists other sites that may work well for you

We comply with the
HONcode standard for health trust worthy
information:
verify
here.
 Tulane Pathology Course
Tulane Pathology Course
Great for this unit
Exact links are always changing
 Adrenal Exhibit
Adrenal Exhibit
Virtual Pathology Museum
University of Connecticut
Mention the normal gross and microscopic anatomy of the adrenal glands, parathyroid glands, and thymus gland. Describe their origins within individuals, and their functions.
Define hypoadrenocorticism, mention the etiologies of the chronic and acute forms, and tell what each looks like clinically. Explain hyperpigmentation in some of these patients, and tell why they are at risk for sudden death.
Describe the etiologies of Cushing's syndrome, from the most to the least common. Tell what symptoms and signs should alert you, the physician, to the possibility of Cushingism. Explain Nelson's syndrome, and why it is becoming uncommon.
Define primary hyperaldosteronism and Conn's syndrome. Distinguish these from secondary hyperaldosteronism. Tell what symptoms and signs point to excess aldosterone, and explain the danger of treating these patients with "safe" diuretics.
Describe in detail the pathogenesis of congenital adrenal hyperplasia, and distinguish the most common salt-retaining and the most common salt-wasting form. Describe the forme fruste that we now believe is very common.
Describe the behavior of carcinomas of the adrenal cortex.
Discuss pheochromocytoma and neuroblastoma with respect to their names, locations, etiologies, catecholamine production, gross and microscopic appearances, clinical picture, and prognosis. Mention the "primitive neuroectodermal tumors" that look like neuroblastomas, and describe "spontaneous cures" of neuroblastoma. Provide an educated guess of how many of your classmates had a "neuroblastoma" at birth.
Describe in some depth the prevalence, etiologies, symptoms, signs and treatment of hyperparathyroidism. Explain how to tell parathyroid hyperplasia from parathyroid adenoma, and why anyone cares. Describe how and when hypoparathyroidism develops, why it is serious, and how to recognize it.
Describe how the size of the thymus gland changes with age. Define thymic hyperplasia and thymoma, tell what they look like, and mention the diseases with which they are associated.
List the components of the important anti-oncogene deletion syndromes MEN I, IIa, and IIb.
Maintain a high index of suspicion for endocrine disease. This lecture ought to scare you.
|
THE ADRENAL CORTEX: "An organ essential to life."
|
{11204} adrenal and its nerve, normal
{11207} adrenal and its nerve, normal {11210} adrenal and its nerve, histology, normal {15035} normal adrenal gland, showing zones (can you figure them out?) |
|
Three "zones":
(1) Zona glomerulosa: mineralocorticoid production. Thin.
(2) Zona fasciculata: glucocorticoid production (?), resting cells (?). Yellow.
(3) Zona reticularis: glucocorticoid production, androgen and estrogen production, grossly darker than outer layers. Brown.
(Mnemonic: Salt, sugar, and sex: the deeper you go, the sweeter it gets.)
Under the microscope, the borders are fairly easy to see.
In newborns, the future adult cortex is a thin layer under the capsule, and most of the gland is "fetal zone". This regresses in a few weeks.
Future pathologists:
The normal adult weight of each adrenal gland is 4 gm.
If an adrenal gland weighs 6 gm or more (without a tumor), it is usually hyperplastic. The stress of the final illness increases the weight of the adrenals, which is why "normal autopsy weight" is sometimes given at 6-8 gm. Most violent suicides have adrenals weighing 9-11 gm (Am. J. Psych. 144: 1214, 1987; confirmed AJFMP 19: 72, 1998.)
* More for future pathologists: A bit of ectopic marrow, a few pigmented cells, or theca cells (especially in menopausal women) are normal.
* Ask a physiologist about the role of dehydroepiandrosterone in health; about 30% of a man's androgens are derived from this, and 90% of a post-menopausal woman's estrogens.
{49431} hyperplasia of adrenal cortex, etiology undisclosed
{09217} adrenal cortical hyperplasia, etiology unknown
* Around 2% of patients have adrenals with black nodules, usually without any evidence of dysfunction.
* CONGENITAL ADRENAL HYPOPLASIA
Two types, both uncommon:
(1) Anencephalic type: thin cortex, no fetal zone
(2) Cytomegalic type (X-linked recessive?): thin cortex composed entirely of large, bizarre cells (foci of such cells are common in normal newborns but regress).
Both present as hypoglycemic seizures in infants. Glucocorticoid replacement saves these children's lives.
ECTOPIC ADRENAL CORTICAL TISSUE (sometimes ectopic adrenal medulla too)
This is most common in the capsule ("capsular extensions") and at the origin of the celiac artery, but it can occur anywhere in the retroperitoneum, or under capsules of liver, kidney, ovary, or testis.
Ectopic adrenal caused problems when surgical adrenalectomy was very popular. ("A new adrenal gland grew back in a different place....")
HYPOADRENOCORTICISM ("Addisonism", etc.): Insufficient glucocorticoid (and usually insufficient mineralocorticoid) production. Review Lancet 361: 1881, 2003.
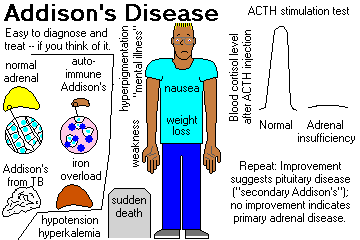
Chronic hypoadrenocorticism (Addison's disease, now regardless of etiology). Troubles start when 80% of the gland tissue is gone. Review Br. Med. J. 312: 1085, 1996.
You need to know the etiologies of chronic hypoadrenocorticism:
Infectious
Most of Dr. Addison's patients had bovine TB of the adrenals (by way of the lymphoid tissue of the duodenum).
{09223} adrenal tuberculosis, gross
{25399} tuberculosis of adrenal, histology
{27257} tuberculosis of adrenal, histology
Worldwide, fungal infections (remember histoplasmosis,
coccidioidomycosis and South American blastomycosis)
and leprosy are important
causes, and now AIDS is too.
Autoimmune
The most prevalent non-iatrogenic cause of Addison's disease in the US today. The adrenal
remnants are typically loaded with lymphocytes, etc., etc. Jack Kennedy suffered
from this illness in his youth, and it was missed for several years (JAMA 201: 115, 1990).
Long-mysterious, it's now clear that most of these patients have autoantibodies against 21-hydroxylase (Lancet 339:
1559, 1992). Presumably this is antibody-dependent cell-mediated
cytotoxicity, as in Hashimoto's disease.
Autoimmune adrenalitis often occurs jointly with Hashimoto's thyroiditis, type I diabetes mellitus,
vitiligo, gluten enteropathy, and/or pernicious anemia (* autoimmune "polyendocrine deficiency
syndrome II", "Schmidt's syndrome", etc.)
* Schmidt's is only the best-known of
a family of autoimmune polyendocrinopathies in which
there are various immune and non-immune disturbances and
a curious inability to keep
candida from infecting mucosal surfaces. Schmidt's etiology is
still obscure, but Type I (2 of 3
hypoparathyroidism, addisonism, mucosal candidiasis; there's often
other autoimmune problems too) has
yielded its secrets. It's an autosomal recessive, known locus APECED, that causes
patients to make an autoantibody against tryptophan
hydroxylase (TPH): Lancet 352: 255, 1998). Surprised that an autoimmune
disease can be simple-mendelian? I was.
Iatrogenic
This results from too-rapid withdrawal of glucocorticoid medication, post-adrenalectomy for breast
cancer or Cushingism, etc., ketoconazole or fluconazole antifungal drug therapy
(Crit. Care Med. 29: 668, 2001), removal of a "non-functioning adenoma"
(rare).
Others: Worth remembering are
{25394} adrenal cortical atrophy (key says "hypoplasia", I doubt this)
NOTE: Hollywood is Hollywood. Evidence that "Lorenzo's oil" benefits adrenoleukodystrophy
patients remains "anecdotal" (Brain & Dev. 14: 409, 1992); it failed controlled studies miserably
(NEJM 329: 745 & 801, 1993; NEJM 330: 1904, 1994; Ann. Neuro. 34: 121 & 169, 1993), and
poisons platelets (NEJM 328: 1126, 1993; Am. J. Hem. 44: 290, 1993; J. Inh. Metab. Dis. 17: 628,
1995) and (at least sometimes) natural-killer lymphocytes (J. Inh. Metab. Dis. 18: 101, 1995).
We've now got two series of dead adrenoleukodystrophy patients who were treated in life with
Lorenzo's oil. It turns out that the stuff doesn't even cross the blood-brain barrier, which is probably
why it doesn't work (Neuroch. Res. 19: 1073, 1995; Ann. Neuro. 36: 741, 1995).
Yet another massive failure: J. Neurol. Neurosurg. Psych. 67: 290, 1999.
Interest continues, and a study from Hopkins (Arch. Neuro. 62: 1073, 2005)
that claimed success in preventing lesions in asymptomatic boys
had no controls and also included other dietary alterations.
ACTH deficiency ("secondary hypoadrenocorticism")
These patients have almost always lost their adenohypophysis and have "panhypopituitarism".
(Treat the whole person.... Caring for a little pituitary dwarf? Don't get focused on the height so that
you forget the likely adrenal insufficiency.... J. Clin. End. Metab. 81: 1693, 1996) Less often, they
have selective, presumably autoimmune, loss of the ACTH-producing cells: Arch. Int. Med. 152:
1705, 1992.
Clinical picture:
"Addisonian" patients show weakness, nausea, and weight loss, and are usually hypotensive
(* 110/70 or less) and have other complaints. Like most endocrine patients, the problems are likely
to appear "emotional".
In primary hypoadrenocorticism, the skin and buccal mucosa will usually be hyperpigmented, due to
increased ACTH (MSH?) -- also look at freckles, nipples, palmar creases, old scars.
Lab studies typically show hyponatremia, hyperkalemia, metabolic acidosis, hypoglycemia, low
serum cortisol, low urinary 17-OH-steroids, and (most important) failure to respond to various
"stimulation tests" by increasing cortisol output.
It is common for these patients to die suddenly and unexpectedly before anyone thinks of
adrenocortical insufficiency. This still happens (Br. Med. J. 312: 1085, 1996).
* Osteoporosis is severe in post-menopausal women with Addisonism, because of loss of adrenal
androgens.
Replacement therapy is life-saving. (And get your patient a syringe of cortisol and an ID bracelet.)
{09371} Addison's disease; pigmentation and vitiligo (mother and daughter)
Selective hypoaldosteronism is rarely due to primary disease of the adrenal cortex. (Clinicians talk
about "hyporeninemic hypoaldosteronism".)
Much more often, the problem is really that the JGA is not producing renin (REE-nin, remember?).
Usually the problem is diabetic arteriolar disease (no surprise); less often, it is one of the diseases of
the renal tubules and/or interstitium.
* These patients have type IV renal tubular acidosis, exhibit normal response to ACTH stimulation
testing, and need a prescription for oral 9α-fludrocortisone.
Acute hypoadrenocorticism ("adrenal apoplexy", "Addisonian crisis"): Sudden collapse, often fatal
(the mechanisms are not fully understood, but it involves opening of the peripheral vasculature and
shock with high cardiac output; consider giving any such patient glucocorticoid: Arch. Surg. 128:
673, 1993.)
Please do not miss this one.
It may result from undiagnosed adrenal insufficiency (iatrogenic, or patients stressed by infection,
surgery, or treatment of concurrent myxedema; see for example J. Traum. 32: 94, 1992), or from
known Addison's disease when extra glucocorticoids are not provided during stress.
Waterhouse-Friderichsen syndrome ("adrenal apoplexy")
features hemorrhage, fibrin thrombi, and sometimes necrosis in the adrenals in a setting
of sepsis. It's not clear whether death is due to adrenal shutdown,
but it's not helping.
This occurs when there is overwhelming sepsis with hemorrhage into, and destruction of, the
adrenals. Patients develop purpura, shock, and die in a few hours.
The etiologic agent is classically N. meningitidis, though staphylococci
(possible new
WF-producing strain NEJM 353: 1245, 2005), pneumococci, and H.
influenzae are other important causes (J. Clin. Path. 57: 208, 2004).
W-F is not rare, and is often overlooked. One group suggests that if your patient in shock does not
have elevated serum cortisol, he or she presumably has W-F. Draw blood, then give 200 or 300 mg
of hydrocortisone (West. J. Med. 150: 582, 1989). Another protocol, for anybody who's septic: Am.
J. Med. 98: 266, 1995.
{24606} Waterhouse-Friderichsen adrenal, gross
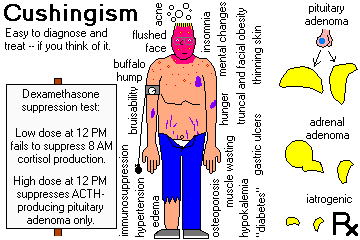
CUSHING'S SYNDROME: too much glucocorticoid. Review NEJM 332: 671, 1995.
1. Iatrogenic (the most common cause nowadays, preventable in part by giving "alternate-day"
glucocorticoid therapy). Of course, the adrenals will be atrophic if glucocorticoids were
administered, hyperplastic if ACTH was administered.
2. ACTH-producing pituitary lesion, usually a basophilic microadenoma ("Cushing's disease",
"pituitary Cushingism")
The adrenals will be nodular, often with one or more large nodules (CT scanners take note -- a
single "adenoma" does not necessarily rule out the need for pituitary surgery).
"Nelson's syndrome" -- rapid enlargement of the pituitary adenoma leading to hyperpigmentation,
blindness and death -- followed adrenalectomy in many of these patients. (Why? It still happens --
sometimes the only way to relieve Cushingism is to remove the adrenals.)
3. Adrenal cortical adenoma or carcinoma ("adrenal Cushingism"); the tumor may be primary, or an
autonomous adrenal tumor may develop after years of "pituitary Cushingism")
4. ACTH- (or CRH-) producing cancers of other organs: oat-cell carcinoma (very well-known),
bronchial and thymic carcinoids (rather common; S. Med. J. 87: 855, 1994; Mayo Clin. Proc. 69: 594, 1994), medullary
thyroid carcinoma, islet cell cancer; other APUDomas. Full-blown Cushingism is rare in oat cell
patients, only because they don't live long enough....
{49441} looks like an oat cell case; adrenal cortex is hyperplastic, and bears a metastasis
* Urocortin is a hormone widely distributed in the nervous system, with CRH-like
activities; it is presently in search of a disease. Both CRF and urocortin are potent anorectic agents.
The newly-discovered "urocortin 3" is also called stresscopin. The truly hardcore
can see J. Clin. Endo. Metab. 90: 4671, 2005.
* 5. Really "primary" adrenal hyperplasia (not due to excess ACTH):
* 6. Cushingism with a burst of cortisol after eating: inappropriate expression of GIP receptors on the
adrenal cortex / adrenal adenoma (NEJM 327: 974, 1992; J. Clin. End. Metab. 81: 3168, 1996;
J. Clin. Endo. Metab. 86: 583, 2001). There are other aberrant receptor
problems as well: J. Clin. Endo. Metab. 88: 416, 2003.
* 7. Recurrent cushingism of pregnancy: Nobody understands it; the adrenal cortex
must over-respond to some non-ACTH hormone made during gestation J. Clin. End. Metab. 81: 15, 1996;
Clin. Endo. 54: 277, 2001.
Both Cushing's disease and glucocorticoid-secreting adenomas are most common in women ages 15
to 45, but can hit anybody, anytime. (* Cushingism in kids and teens: NEJM 331: 629, 1994).
Symptoms and signs that should alert you to possible Cushingism:
{09367} Cushingism, face
* Future pathologists: Heavy negative feedback on pituitary basophilic ACTH-producing cells
produces "Crooke's hyaline change".
PRIMARY HYPERALDOSTERONISM ("low-renin hyperaldosteronism"): too much
mineralocorticoid (review: Postgrad. Med. 95(4): 199, March 1994;
NEJM 339: 1820, 1999; Lancet 353: 1341, 1999; Surg. Clin. N.A.
84: 887, 2004)
This results from "idiopathic" adrenal hyperplasia, or an adrenal adenoma.
This is important as a cause of surgically-correctable high blood pressure. Maybe 0.5% of
hypertensives have primary hyperaldosteronism.
Classically, patients exhibit hypokalemia, alkalosis, and low renin, and a failure of plasma aldosterone levels to
increase significantly when the patient goes from supine to standing position.
Surprisingly, these patients do not have edema. (The effects of
aldosterone in hanging onto body salt is overridden
by atrial natriuretic peptide.)
Trap: These patients can die from hypokalemia if you give them thiazide diuretics to treat their high
blood pressure.
Today, we screen by looking for the plasma aldosterone / plasma renin activity
ratio. Around 5-10% of hypertensives have elevated levels,
and many of these people will indeed have an aldosteronoma that can
be removed (Am. J. Med. Sci. 324: 227, 2002); the rest will usually
have hyperplastic cortices.
The most familiarr cause is an "autonomous" adrenal cortical adenoma (Conn's syndrome), often very small.
It produces aldosterone (* rare Conn-omas produce DOC instead).
You'll clinch the diagnosis by sampling aldosterone levels in the
adrenal veins (J. Clin. End. Metab. 86: 1066, 2001).
Surgery is curative (Ann. Surg.
219: 347, 1994; Postgrad. Med. 95(4): 199, Mar. 1994); it is now
routinely done via laparoscope (review J. Urol. 169: 32, 2003).
The rest of the patients have "idiopathic hyperaldosteronism", with normal or hyperplastic adrenals.
These patients get spironolactone. Not surprisingly, the borderland between
these and the adenomas is blurry (Surgery 106: 1161, 1990); probably it's best
to operate only if the hypertension is unsuppressible medically like a Conn-oma.
A few patients have glucocorticoid-correctable hyperaldosteronism and hypertension. This is
transmitted autosomal-dominant. It is now clear that the problem is a chimeric beta-hydroxylase/aldosterone synthetase gene
(Nature 355: 262, 1992; Lancet 339: 1024, 1992;
screening kids Arch. Dis. Child. 71: 40, 1994; J. Urol. 154: 510, 1995).
Update J. Clin. Endo. Metab. 87: 3187, 2002. When the cell is told by
ACTH to make cortisol, it pumps out huge amounts of aldosterone, too. (Thinkers: Giving a tiny
amount of exogenous glucocorticoid solves the problem. How? If you can't answer this, consider
taking year I over again.)
* Hypertension from a mutated aldosterone receptor stuck in the "on" position:
Science 289: 119, 2000.
* Another cause is "apparent mineralocorticoid excess syndrome", a lack of
11-β-hydroxysteroid dehydrogenase type 2,
which turns cortisol to cortisone in the renal tubules; cortisol ends up overstimulating
the mineralocorticoid receptors. The forme fruste may be a common contributor
to "idiopathic" low-renin hypertension even with normal potassium.
See J. Clin. Endo. Metab. 86: 1247, 2001; Lancet 353: 1341, 1999. Yet another is
a 21-deoxyaldosteronoma (J. Clin. End. Metab. 80: 737, 1995). Rarely an ovarian cancer produces
aldosterone (series Arch. Int. Med. 156: 1190, 1996).
Secondary aldosteronism is much more common. It is part of the picture in CHF, cirrhosis,
nephrotic syndrome, Goldblatt hypertension, and other common problems.
Don't forget Bartter's hypokalemia (vessels are insensitive to angiotensin and/or the sodium pump in
the ascending loop of Henle doesn't work -- Hosp. Pract. 29(5): 103, 1994.
Don't confuse this with salt-retaining congenital adrenal hyperplasia (see below).
CONGENITAL ADRENAL HYPERPLASIA: autosomal-recessive virilization syndromes that, in
their most severe forms, affect young children.
Deficiencies (mild or severe) of the various
enzymes required to synthesize cortisol
result in decreased production of cortisol
and other hormones.
This results in increased ACTH, with resulting adrenal cortical hyperplasia.
Steroid precursors are shunted into the production of abnormally large amounts of
the androgen androstenedione
(ambiguous genitalia in girls, "infant Hercules" and Leydig cell nodules in boys, etc.)
Remember these two types (there are at least six others):
21-hydroxylase deficiency (most common): no cortisol, aldosterone, or DOC, hence salt wasting.
This gene is inside the HLA locus. Review J. Clin. Endo. Metab. 88:
2624, 2003.
11-betahydroxylase deficiency: huge amounts of DOC, causing salt retention and high blood
pressure (molecular biology of the gene Proc. Nat. Acad. Sci.
90: 4552, 1993).
Full-blown congenital adrenal hyperplasia is a devastating illness, especially for women.
Mild variants of these syndromes (i.e., relatively ineffective enzymes -- especially 21-hydroxylase
deficiency) are probably widespread -- causing, for example, amenorrhea in girls or hirsutism in
older women.
It's important to find these people because a little dexamethasone given daily will greatly improve
the internal milieu.
To test your female patient with amenorrhea or hirsutism, administer ACTH and measure plasma
17-hydroxyprogesterone one hour later. It will be elevated if your patient has even mild
21-hydroxylase deficiency.
ADRENAL CORTICAL ADENOMAS
These are round, yellow (like the adrenal cortex) nodules. (* Purists call them "nodular
hyperplasia" if the surrounding cortex is at all lumpy-bumpy).
Adrenal cortical adenomas are surprises at around 2% of autopsies and abdominal CT scans. They
can cause Cushing's syndrome, Conn's syndrome, or virilization -- but the vast majority seem to
produce nothing.
They are commonly discovered on CT scans too, and clinicians are learning to ignore small adrenal
masses ("incidentalomas", so long as there is no evidence of steroid or catecholamine over-production).
* Leave discussions of the arcana of these common lesions
to us. This includes "spironolactone bodies" (pink scroll-like things,
also found in the ZG of folks taking spironolactone), "black adenomas", and
much more.
"Adrenal cysts" seen on scans have unpredictable pathology: Cancer 101: 1537, 2004.
{09220} adrenal cortical adenoma, gross
* Adrenal myelolipoma: a metaplasia-choristoma made of bone marrow. They can be big
but are generally harmless. You can see bone
marrow in adrenal cortical hyperplasia too.
{25412} adrenal myelolipoma, gross
ADRENAL CORTICAL CARCINOMA (Am. J. Clin. Path. 105: 76,
1996; J. Urol. 169: 5, 2003)
These are rare cancers that are often lethal. The majority
are hormonally active.
Future pathologists: These are usually obviously malignant,
grossly and microscopically, with ten or more mitotic figures per high
power field.
* Mixed endocrine syndromes usually mean cancer. Adrenal tumors that feminize, or that produce
androgens without glucocorticoids, are most often malignant. Mitotane, an analogue of the old-fashioned insecticide DDT, is the
classic
mainstay of therapy.
* Future pathologists: Use electron microscope to find tubulovesicular mitochondria, which are
characteristic of adrenal cortex. Criteria for malignancy have been developed;
since this cancer is not particularly treatable, their usefulness
is limited.
{24087} adrenal cortical carcinoma, gross
NOTE: Cancer in the adrenals is usually metastatic carcinoma. Half of all lung cancers metastasize
to the adrenals. Adrenal insufficiency often results, but is usually missed clinically.
THE ADRENAL MEDULLA: "An organ not essential to life".
Around 10% of the normal adrenal by weight.
The source of "adrenalin" (epinephrine, also norepinephrine). At autopsy it is gray, unless it has
autolyzed.
The only diseases are two tumors that may arise here or at the other chromaffin tissue masses --
pheochromocytoma (well-differentiated, adults) and neuroblastoma (poorly-differentiated, children).
* The story of the old adrenal-to-brain transplant for parkinsonism: Mayo Clin. Proc. 65: 305, 1990.
PHEOCHROMOCYTOMA ("paraganglioma", "pheo", "ten percent tumor";
big NIH consensus review Ann. Int. Med. 134: 315, 2001).
This tumor is named for its colorful reaction in fixatives containing chromic acid salts.
Pheochromocytomas secrete norepinephrine (most common) and/or epinephrine (usually less, * and
often other things, including dopamine, serotonin, ACTH, somatostatin, neuropeptide Y, and/or
VIP; Cancer Res. 49: 7010, 1990).
The infamous paroxysms of extreme hypertension, accompanied by sweating, headache,
and other autonomic disturbances, probably result from physical compression and/or ischemia
of the "pheo".
Even a tiny (1 gm) benign pheochromocytoma can make a person very sick and will eventually
cause death.
Most (50-90%, estimates vary) occur in the adrenal medulla (you may learn "10% are extra-adrenal"). Other sites are the
organs of Zuckerkandl (i.e., the little nubbins of chromaffin tissue
at the origin of the inferior mesenteric artery and/or aortic bifurcation), paravertebral sympathetic
chain, urinary bladder (patients get terrible headaches whenever they urinate), or "paraganglia" such
as the carotid body. Ten percent occur in children.
Ten percent of pheo cases involve both adrenals, and many of these are also among the 10% that are
familial. Syndromes include:
You will often be reminded of the MEA ("MEN", "multiple endocrine adenoma/neoplasia
syndromes") -- common autosomal dominant conditions that predispose patients to certain
endocrine tumors. Pre-natal diagnosis is available for these tumor-gene syndromes.
Learn them now:
MEN I: PPP (Wermer's syndrome), * protein menin, on 11q13
Parathyroid adenoma(s) (1 or, often, more glands) /
"chief cell hyperplasia" (i.e., all four glands):
NEJM 321: 213 1989)
Pituitary adenoma (anterior)
Pancreatic islet cell adenoma (gastrinoma)
MEN IIa: PAC (Sipple's syndrome); the RET gene (Nature 367: 315, 375,
377 & 378, 1994; NEJM 335: 943, 1996; J. Clin. End. Metab. 81: 2711, 1996; screening for the
gene J. Clin. Endo. Met. 78: 1261, 1994 and
Mayo Clin. Proc. 72 430, 1997; new alleles keep appearing J. Clin.
Endo. Metab. 89: 4142, 2004;
surveillance J. Clin. End.
Metab. 82: 897, 1997).
Parathyroid adenoma(s) (1 or, often, more glands) / "chief cell hyperplasia"
Adrenal medullary tumor (pheochromocytoma) or hyperplasia
Calcitonin-producing hyperplasia-carcinoma of thyroid
MEN IIb:
Similar to MEN IIa; the patients have Marfanoid body habitus and mucosal (ganglio)neuromas
(bumps on the edges of their tongues and elsewhere), and probably will not have parathyroid
problems. Same locus, different allele (Nature 1994, see above.)
Grossly, pheos are very bloody (because they are very vascular), and often show fibrosis,
calcification, cystic change, or even * fatty change (?!)
Microscopically, pheos resemble adrenal medulla.
* Special stains are available that show norepinephrine and/or epinephrine in granules (* future
pathologists: aqueous fixation washes them out.) Contrary to what anybody else may
have told you, the granule morphology means nothing: Br. J. Surg. 85: 1681, 1998.
For pheos, there are no histologic criteria for malignancy, not even vascular invasion. The honest
pathologist cannot predict the tumor's behavior. Although 10% of these tumors can metastasize, to
prove malignancy you must find pheo in a location where it could not have arisen.
Five-year survival rate with malignant pheo is around 50%.
* "Pheo balls" are hyaline spheres that can be very big. You can see them
in most normal medullas if you look hard enough.
Nobody knows for sure what they are, but
they seem to stain for vimentin and glial filament acid protein
(Am. J. Clin. Path. 102: 163, 1994,
which deals with how to spot bits of neuroblastoma
which may be present on pheo's.) Fun to know: They are acid-fast and
autofluorescent!
More fun: A pheo or neuroblastoma, as a frozen section, exposed to formalin, fluoresces
yellow-green from the catecholamines getting altered.
Regardless of location and appearance, the patients will report anxiety,
headache, palpitations, "panic attacks", sweating, dizziness,
etc. (Again, you may suspect the basic problem is emotional. "Pheo is a great imitator.")
"Textbook" pheochromocytoma patients have paroxysms of severe hypertension. Actually, the
majority show sustained high blood pressure.
Pheochromocytoma is present in fewer than 1% of people with high blood pressure, but it's a
diagnosis you don't want to miss.
Pheos are still often missed clinically (Am. J. Surg. 179: 212, 2000)
and are all-too-familiar surprises at autopsy (Lancet 335: 1189,
1990). The patients typically had been told they had "benign essential hypertension" and "emotional
problems".
In addition to causing bad high blood pressure and all that goes with it, high levels of circulating
catecholamines can directly (though reversibly) damage the myocardium
can cause coronary spasm, and can play
havoc with smooth muscle (renal arteries, bowel, brain, etc. -- angiographers
may report "vasculitis".)
Screening tests for pheos detect increased amounts of catecholamines or their metabolites in blood or
urine.
The classic screen for pheos (24 hour urinary vanillylmandelic acid -- VMA) is being superseded by
more sensitive and specific tests.
Today's "most sensitive screen" is the plasma free metanephrine assay (Ann. Int. Med. 134:
315, 2001; Arch. Int. Med. 160: 2521, 2000; JAMA 287: 1427, 2002).
There's a radioisotope scanner/treatment for pheo and neuroblastoma -- I-131 labeled
metaiodobenzylguanidine (MIBG); it's not very sensitive for diagnosis
(J. Clin. Endo. Metab. 86: 685, 2001).
* Finding the hidden pheo using novel techniques, including 6-[18F]-fluorodopamine:
J. Clin. Endo. Metab. 86: 3641, 2001.
* The Mayo crew examines how much it would cost to screen every hypertensive
patient for pheo using each of three methods, and simply states that
spending $50,000 or $100,000 per patient found isn't worth it; I disagree
(J. Clin. Endo. Metab. 89: 2859, 2004), and certainly you need to
work up the young ones, the ones with headaches, and the ones with "nerves".
Treatment is surgical, with very careful management of fluid status and blood pressure before and
after surgery (J. Urol. 161: 764, 1999). The anesthesiologist, of
course, has an extra challenge (Anesth. Analg. 91: 1118, 2000).
And surgeons must be careful manipulating the tumor!
Adrenal sparing surgery, i.e., let's leave a bit of cortex behind:
Br. J. Surg. 86: 94, 1999. Today, it's likely that the tumor
will be removed successfully via the laparoscope (Urol. Clin. N.A. 28: 97, 2001).
* Injecting epinephrine to fake a pheo: JAMA 266: 1553, 1991 (weird!).
{20316} pheochromocytoma in adrenal, gross
NEUROBLASTOMA (CA 45: 179, 1995,
Ped. Clin. NA 44: 919, 1997.)
The second commonest solid pediatric cancer (after Wilms tumor). It is derived from primitive
nerve elements (* and the cells will always grow neurites, at least in tissue culture). Discovered by
Virchow.
A majority of neuroblastomas arise in or near the adrenals.
Grossly, neuroblastomas are soft, white tumors.
Portions often undergo dystrophic calcification (which helps the radiologist make the diagnosis.)
The tumor eventually metastasizes widely. "Blueberry muffin baby" is a repulsive, classic
description for a neuroblastoma patient with multiple skin metastases.
Histologically, neuroblastoma is a tumor of "small blue cells" (i.e.,
cancers with small, fairly-uniform cells with scanty cytoplasm). Often (but
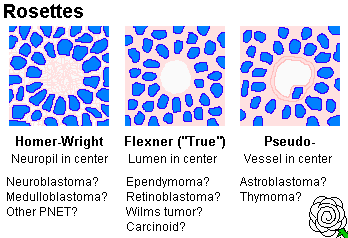
not always) the cells are arranged in "rosettes" (recalling neural tubes) around a tangle of neurites
(* "Homer Wright rosettes", pretty-much diagnostic).
EM shows neurosecretory granules and * sometimes
neurites too. (* Purists: True rosettes surround a hole. Think of ependymomas or retinoblastomas.)
* Future pathologists: Immunohistochemistry helps differentiate this from other "tumors of small
blue cells" (the LEMON family), and will also help you recognize their nondescript cells in bone
marrow. The better-differentiated neuroblastomas
are likely to stain positive for neuron specific enolase, S-100,
and/or synaptophysin, but the more primitive ones will be negative for everything.
In tissue culture, neuroblastoma cells sprout neurites almost at once.
Grading of the tumor is based on mitotic figure counts, with karyorrhexis being taken into account
nowadays also: Cancer 77: 1582, 1996 updates the older Shimada system.
The most recent update is the "International Neuroblastoma Pathology Committee"
system (Cancer 86: 364, 1999; Cancer 92: 2451 & 2699, 2002; Cancer 94:
1574, 2002).
Most neuroblastomas produce catecholamines, resulting in elevated urinary metabolites. (* They
also produce certain characteristic protein markers, etc., etc.)
The classic test involves checking urine for homovanillic acid (HVA) and vanillylmandelic acid
(VMA).
Japan screens all babies at six months of age (Lancet 2: 152, 1988); skeptical Brits suggest that
they find only regressing tumors (Lancet 337: 344, 1991). The Germans
now screen at one year and find it lot of cases, but I couldn't tell
whether they're
saving lives (J. Clin. Onc. 17: 1200, 1999 -- anybody want to do a lab
presentation on this?). Now the Japanese are doubting whether they've saved
any lives, either (Canc. Caus. Contr. 9: 631, 1998; "we haven't" J. Ped. Surg. 37: 949, 2002).
Neuroblastoma screening has not
caught on in the US in the "managed care era"; both big studies indicate it's a
bad
idea NEJM 346: 1041 & 1047, 2002.
There are also a variety of curious paraneoplastic syndromes that result from neuroblastomas,
including neurodegenerative disorders similar to those in oat-cell carcinoma.
Prognosis in neuroblastoma:
And prognosis depends on differentiation:
In a baby, the tumor is likely to regress/differentiate/mature (to a stroma-rich
GANGLIONEUROBLASTOMA or a thoroughly benign
GANGLIONEUROMA; Br. J. Surg. 83: 263, 1996). In 2%
of autopsies on infants dying of unrelated causes, there is a neuroblastoma-like "incidentaloma".
Obviously most of these cure themselves. We wish we knew exactly why/how this happens.
* The process begins with the appearance of S100-positive Schwann cells, which are from outside the
tumor. If the tumor is to self-cure, it must be near-triploid and have intact chromosome 1 (NEJM
334: 1505 & 1537, 1996).
{24716} neuroblastoma, histology, good rosettes
In toddlers, spontaneous remission is less likely, but even metastatic disease is often
cured by chemotherapy.
In older kids and adults, neuroblastomas grow slower but seldom
self-cure or respond well to therapy (Cancer 79: 2028, 1997).
Oncogenes, especially N-myc, are clearly important in the origin and progression of neuroblastoma.
N-myc amplification is common and is a bad sign as far as final outcome.
It correlates with high mitotic rates and * large nucleoli (Cancer 103:
174, 2005).
* Neuroblastomas also commonly have a break in the short arm of chromosome 1, leading to loss of
a (presumed) anti-oncogene in the region 1p32-36 (Science 254: 1153, 1991; bad prognosis NEJM
334: 225, 1996, means it won't mature NEJM 334: 1505, 1996).
Retinoblastomas (cones of the eye), medulloblastomas (cerebellum),
pinealoblastomas (pineal), and adult neuroblastomas (lots of places) are related pediatric tumors that
look like neuroblastomas microscopically.
* The tendency today is to call these "primitive neuroectodermal tumors", despite obvious
differences in their basic biology.
Adult neuroblastomas share with Ewing's sarcoma the characteristic translocation t(11:22)(q24;q12)
and a distinctive tumor antigen (Cancer 67: 1886, 1991).
* Neuroblastoma of the olfactory epithelium is a special entity.
INTRODUCTION TO ADRENAL TESTING
The aphorism -- "A physician is only as good as his/her index of suspicion" -- is especially applicable to
endocrine disease. As an alert clinician, you will often suspect adrenal gland problems and will
want to order sensitive tests.
Some classic cases:
Order a rapid ACTH ("cosyntropin") stimulation test
Order serum cortisol determinations at 8 AM and 8 PM (circadian rhythm is always lost in
Cushingism), plus a low-dose dexamethasone suppression test, or just order one (or maybe two
or three) 24 hr urinary free
cortisol assays.
Screen with a serum aldosterone/renin ratio. If high, measure
urine aldosterone on a high-salt diet and/or
see whether you can suppress the aldosterone using fludrocortisone and/or
check to see if plasma aldosterone fails to increase on standing up
and/or
consider performing a saline infusion aldosterone-suppression test
and/or consider a CT scan. Nobody really knows what's best.
Administer ACTH and measure blood 17-OH-progesterone.
Check serum/urine catecholamines and/or metabolites, ask your lab.
Of course, only some of these patients are endocrine cases. As a rule, meaningful hormone assays
are performed under conditions of attempted stimulation (if you suspect deficiency) or attempted
suppression (if you suspect over-production). If your screening tests are positive, or if you have any
doubts, get consultation. (One problem is that reliable, cheap plasma ACTH assays are still not routinely available.)
If you diagnose endocrine disease that is not present, the patient gets lifelong medication,
unnecessary surgery, or unnecessary radiation. If you fail to diagnose a disease that is present, the
patient is likely to die of a disease that might have had an excellent prognosis. (Suicide is
common among patients with untreated Cushing's syndrome.) If you make the correct diagnosis, the treatment
of endocrine disease is very satisfying to physician and patient alike -- because it works.
The parathyroids arise from the third and fourth branchial clefts. They are each
3-4 mm across and
weigh maybe 35 mg each (there is no consensus about "ideal total weight").
* They were first discovered by Richard Owen in 1850 in a rhinoceros in the London
zoo. Virchow (who else?) discovered them in humans.
Future surgeons: The key to telling a parathyroid gland / tumor at surgery
is that (unlike fat, thyroid nubbins, or lymph nodes), a droplet of blood
will ooze up when the gland is pricked by your scalpel blade. Why?
Textbooks show four. Most people have 3 or 4 parathyroids, less often
5 or even 6.
Unusual locations (especially for the lower pair) are common and explainable embryologically.
"Ectopic" parathyroid glands may be found in the carotid sheaths, behind the esophagus, in the
anterior mediastinum, in the thyroid gland, etc. The new sestamibi scan
has made finding these much easier. Cysts are
uncommon but do occur (J. Otol. 18: 311, 1990).
Cell types in the normal glands:
Chief cells: typical hormone-secreting endocrine cells
Oxyphils: large, pink-staining cells that appear after puberty and occur in clusters. (By EM, these
are seen to be packed with mitochondria, like Hürthle cells. They do not contain secretory
granules, and the mitochondria are probably not metabolically active.
Oxyphils are more numerous in older people.)
"Water-clear cells" ("wasserhelle" cells): seen in some parathyroids. (They are full of glycogen, and
their functional status is uncertain.)
Fat cells: after puberty. "Fatty ingrowth", if you like. Parathyroid hormone is the major regulator of calcium homeostasis in humans.
Its production-secretion is regulated by serum calcium levels. I suggest measuring both. With high PTH, high calcium, and low phosphorus,
you have your diagnosis. Other labs aren't so useful.
(Did you know that urinary cyclic AMP is a good estimate of
parathyroid hormone levels?)
Effects:
Kidney: Promotes conversion of 25-OH-D3 to the very active 1,25-OH-D3 that increases calcium
absorption from the gut.
Promotes resorption of calcium from the glomerular filtrate, and promotes loss of phosphate.
This effect is mediated by activation of adenylate cyclase.
Serum parathyroid levels are accurately reflected by measuring urinary c-AMP concentrations.
Bone: Promotes resorption of calcium by osteoclasts (via activation of adenylate cyclase.)
Increased levels of parathyroid hormone causes proliferation of osteoclasts. (Finding of an
osteoclast in a section of bone from an adult usually means hyperparathyroidism.)
Gut: Promotes calcium absorption (indirect effect, via vitamin D activation).
HYPERPARATHYROIDISM ("stone and bone disease"; review Mayo Clin. Proc. 77: 87, 2002)
Primary hyperparathyroidism: Due to disease of the parathyroid glands.
80%... parathyroid "adenoma"
<1%...
iatrogenic seeding causing lots of little glands: Hum. Path. 21: 234, 1990, (* by my teacher,
Dr. David Roxe); Surgery 116: 111, 1994.
Some people may also use this term
to include hypercalcemia caused by
production of parathyroid-hormone-like activity (PTH-rP)
by cancers, notably squamous lung cancer.) Today the term
"pseudohyperparathyroidism" is preferred.
Primary hyperthyroidism is a common clinical problem. About 1 person in 1000 will need
parathyroid surgery during his or her lifetime.
Calcium rises because of enhanced GI absorption
and renal reabsorption. There is also a tiny contribution from increased osteoclastic activity
(Am. J. Med. Sci. 320: 334, 2000).
Symptoms and signs:
Elevated serum calcium on routine screening (probably the commonest presentation today)
Mental changes (depression, psychosis; Am. J. Med. 101: 111, 1996)
Kidney stones (the commonest presentation in the past)
Nephrocalcinosis (metastatic calcification in the tubular basement membranes with eventual damage
to the tubules)
Bone changes (first osteomalacia, then widespread involvement of the skeleton by increased osteoclastic
activity especially in the centers of trabeculae;
finally cystic lesions variously known as "osteitis fibrosa cystica",
"von Recklinghausen's disease of bone", or "brown tumors."
All this heals after the hyperparathyroidism is fixed.)
Just not feeling right. Hypercalcemia makes you fatigued
before anything else. People who eventually turn out to have
primary hyperparathyroidism are much more likely to have been missing
a lot of work "for being sick" for several years prior to
diagnosis (BMJ 317: 848, 1998.) After surgery, "asymptomatic"
people with parathyroid adenomas feel a lot better (Surgery 128:
1013, 2000).
Resorption of the tufts of the phalanges on x-ray
* "Band keratopathy" of Bowman's membrane in the cornea
* Loss of lamina dura on dental x-rays
Gastric ulcers (5%; hypercalcemia from any cause enhances gastrin secretion)
Hypertension (50%, cured by parathyroid surgery only if the kidney is undamaged)
Pancreatitis (occasionally)
Pseudogout (occasionally)
* NMJ problems (denervation-atrophy picture on muscle biopsy)
* Skin ulcers (following metastatic calcification of vessels: Arch. Path. Lab. Med. 114: 482, 1990).
Labs:
high serum calcium
low serum phosphate
high 24-hour urine calcium excretion (i.e., you absorb a lot more through your gut)
high urinary cAMP
high serum parathyroid hormone (PTH; remember you can choose between N-terminal and C-terminal assays) for your screening
PARATHYROID ADENOMA
The commonest cause of primary hyperparathyroidism.
* In the mid-1980's, there was a fad notion that these all represented "nodular hyperplasia". Newer
genetic studies have shown these tumors truly are monoclonal.
These tumors are most common in older women, but may occur in anyone. They average around a
gram but are sometimes bigger. * I've seen several at autopsy that were probably non-functional.
The basic biology very often involves translocations that deregulate the oncogene PRAD-1 (bcl-1, cyclin D1, a cell-cycle regulator; Nature
350: 512, 1991, more since).
The surgeon finds three normal glands and an adenoma that is easily removed (10% are in an
"ectopic location.)
In the late 1990's, parathyroid surgery was revolutionized by the introduction
of the Tc99 sestamibi scan to locate the lesion(s)
preoperatively, and intraoperative radioguidance (Arch. Surg. 135: 481 &
550 & 844 & 1461, 2000;
Ann. Surg. 23: 31 & 331 732, 2000;
J. Am. Coll. Surg. 190: 540, 2000; Surg. Clin. N.A. 80: 1399, 2000;
Surgery 129: 720, 2001;
Arch. Surg. 137: 659, 2002). The sestamibi scan is very specific.
The surgeon can draw a iPTH (intact PTH) level
before and soon after (half life is five minutes)
excising the suspected lesion; if it drops 50% or more,
supposedly your operation was a success. (See Arch. Surg. 136:
536, 2001; Surgery 128: 1029, 2000.)
Nowadays, it seems quite clear that iPTH it can indeed be used as a "chemical
frozen section", and satellite labs are appearing in operating suites.
Pathologists
will be doing a lot fewer frozen sections / imprint cytologies on the cases (Arch. Path. Lab. Med. 127: 1424, 2003; shucks,
I used
to love doing these).
It's usually an outpatient procedure done under a local anesthetic.
* Imprint cytology of the parathyroid and other neck structures
at surgery: Arch. Path. Lab. Med. 127: 64, 2003; Am. J. Clin. Path. 118:
895, 2002.
The adenoma often has a rim of compressed normal gland at the edge.
Most adenomas contain few if any adipocytes, and most of the cells do not have
intracytoplasmic fat.
An adenoma can be composed of any of the three
kinds cells (chiefs, oxyphils, waterclears).
As you would expect, parathyroid adenomas (even in people with no family history) often lack
11q13 (the MEN-I locus).
* Every once in a while, there is so much fat in a parathyroid adenoma
that it looks like a lipoma, i.e., the infamous "lipoadenoma".
{10827} parathyroid adenoma, histology
PARATHYROID CARCINOMA (Cancer 100: 900, 2004)
A rare cause of primary hyperparathyroidism.
By definition, this cancer arises in a parathyroid gland and produces parathyroid hormone.
* Loss of parafibromin nuclear reactivity as a marker for malignancy:
Clin. Canc. Res. 10: 6629, 2004.
These cancers are not very aggressive. About a third are cured with simple excision,
another third recur and require re-operation for cure, and only a third eventaully
metastasize and ultimately cause death, usually from
refractory hypercalcemia.
* Loss of Rb seems to be the rule for parathyroid carcinoma, in contrast to parathyroid adenomas:
NEJM 330: 757, 1994; J. Clin. End. Metab. 81: 3194, 1996. No surprise; it will probably prove
useful in telling the lesions apart. A familial syndrome involves mutant HRPT2 (parafibromin: NEJM 349: 1722, 2003).
PARATHYROID HYPERPLASIA
The second most important cause of primary hyperparathyroidism.
All four glands are big, for no obvious reason. Even though this is "a different
disease from adenomas", the masses are often clonal, and (in the case of chief-cell
hyperplasia) the same genes put you at risk.
This may occur in anyone, but is suspicious for one of the multiple endocrine neoplasia (multiple
endocrine adenomatosis) syndromes. See below.
Hyperplastic glands usually lack the usual fat cells.
Chief cell hyperplasia is the common kind. It raises the possibility
of MEN I or MEN II. Today's pathologists may tentatively distinguish a
hyperplastic gland from an adenoma because hyperplastic chief cells
still contain intracellular fat (uh, supposedly).
Water-clear hyperplasia is a different disease with the same
symptoms. All four glands are quite big,
probably because there is much non-functioning cytoplasm
in the clear cells. This time, there is no intracellular lipid but plenty of glycogen.
{27260} parathyroid hyperplasia (arrow sign helps)
In parathyroid hyperplasia, the bulk of the parathyroid tissue must be removed, leaving a small amount behind. (Sometimes a
small amount of parathyroid tissue gets transplanted to the forearm, for future whittling.)
NOTE: The honest pathologist CANNOT distinguish a hyperplastic gland from an adenoma! The
surgeon must send samples of two glands. (Why?)
Secondary hyperparathyroidism: Parathyroid hyperplasia due to hypocalcemia (or
hyperphosphatemia, or hPTH resistance, or vitamin D deficiency, or vitamin D resistance) from
some other cause, usually renal failure (less often malabsorption or malnutrition).
In people
who are vitamin D deficient, the extra parathyroid hormone keeps calcium levels normal
(J. Clin. Endo. Metab. 85: 4125, 2000; Am. J. Med. Sci. 319:
380, 2000; remember there's plenty
of this in the US).
Less common causes are intestinal malabsorption, calcitonin-producing tumors (i.e., medullary
carcinoma of the thyroid) and rickets (low serum phosphate, in contrast to renal failure, in which
serum phosphate levels are high.)
Serum calcium is low-normal.
Bone disease (as in primary hyperparathyroidism, but now called "renal osteo-dystrophy") is a big
problem. Some patients may need partial parathyroidectomy to control it.
Today's patient with secondary hyperparathyroidism of renal origin
will probably be controlled
adequately with oral, or perhaps intravenous, calcitriol (Am. J. Med. Sci. 320:
100 & 107, 2000).
Tertiary hyperparathyroidism: Hypercalcemia develops in a setting of secondary
hyperparathyroidism.
One or more glands has "become autonomous" and overproduces parathyroid hormone. Probably
this means it has lost the MEN-I anti-oncogene on chromosome 11 (J. Clin. End. Met. 76: 139,
1993). Thankfully rare. Other hyperparathyroidism syndromes:
The mutation is in CaSR, the calcium sensing receptor (Clin. End.
50: 537, 1999; Am. J. Hum. Genet. 64: 189, 1999),
which tells the nucleus what the plasma calcium level is. Parathyroid hormone
is overproduced as a result.
* Two doses gives neonatal severe primary hypercalcemia requiring
total parathyroidectomy.
* Other mutations give a familial hypocalcemia (J. Clin. Endocrin. Metab.
84: 363, 1999), i.e., in these "activating"
mutations, the receptor is stuck in the "on"-position. An autoantibody
that causes the same problem: NEJM 351: 362, 2004.
Order a calcium-creatinine clearance ratio, which will be very low (less than .01) in these patients.
HYPERCALCEMIA: Differential diagnosis for beginners. Review Postgrad. Med. 115: 69, 2004.
HYPOPARATHYROIDISM
The most common cause is iatrogenic (following thyroid surgery).
Next is autoimmunity (remember the syndrome with addisonism, ectodermal dysplasia, and mucosal candida? --
J. Clin. Endo. Metab. 88: 4602, 2003).
In both familial and sporadic cases, the autoantigen is often CaSR (J. Clin. Inv. 97:
910, 1996; update
J. Clin. Endo. Metab. 89: 4484, 2004).
You also remember DiGeorge's syndrome. * Zebras: Kearns-Sayre / mitochondriopathy (J. Clin. Endo. Metab. 83:
125, 1998), Wilson's, Riedel's struma.
Symptoms and signs of hypocalcemia begin with mental changes, circumoral paraesthesia,
Chvostek's sign, Trousseau's sign, and progress to carpopedal spasm, convulsions, tetany. Check for
cataracts, too; nobody knows why they tend to occur when ionized calcium is low.
The diagnosis is confirmed by finding low serum calcium and high serum phosphate.
* Treatment includes vitamin D and calcium gluconate cookies (lifelong.)
Injectable parathormone is now becoming
available (J. Clin. Endo. Metab. 88: 4214, 2003).
Pseudohypoparathyroidism Some curious disorders. In each,
there is an inability to carry the parathyroid hormone receptor signal to the
cell's machinery.
(Proc. Nat. Acad. Sci. 95:
10038 & 11798 & 15475, 1998; Am. J. Med. Genet. 77: 261, 1998;
J. Clin. End. Metab. 81: 1660, 1996).
As you'd expect, serum calcium runs low, phosphate runs high, and
(except in type II)
urinary cAMP fails to rise in response to parathyroid hormone administration.
In Pseudohypoparathyroidism type Ia,
there is a mutation
of a portion of the GNAS1 gene that codes
for Gsα. There is a striking imprinting effect.
Hence, if you inherited a bad allele from your mother, you get
resistance to parathyroid hormone (marked), plus some resistance to other
endocrine hormones. Plus, you get the bony deformities
("Albright's hereditary osteodystrophy"), with
short stature, short fingers, and a round face.
If you inherited a bad allele from your father, you get
ONLY the bony deformities ("pseudopseudohypoparathyroidism").
* Certain bad alleles inherited from Dad produce the thankfully-rare
progressive osseous heteroplasia, in which skin and muscle transform
into bone (NEJM 346: 99 & 128, 2002).
In Pseudohypoparathyroidism type Ib,
the bones are formed normally. The kidney is resistant to the effects
of parathyroid hormone and there is milder resistance to TSH as in type Ia.
The mutated gene (* STX16 / syntaxin 16)
is adjacent to the GNAS1 locus; it's recently been identified
as a portion of the complex that's responsible for its being imprinted
(J. Clin. Inv. 1255: 112, 2003). It can only be expressed
if it is inherited
from Mom.
In pseudohypoparathyroidism type Ic, the alpha subunit is normal
but there is the osteodystrophy. Too rare to know anything more yet; I'm betting
on a different allele at the GNAS1 locus.
In Pseudohypoparathyroidism type II, there is resistance to the
effects of parathyroid hormone in bone and kidney, and some bone deformities.
However, the urinary cAMP response to PTH challenge is normal, and
The GNAS1 locus is not involved (i.e., there's a problem with the signal distal to cAMP).
The genetics remains obscure.
* If you lack both copies of the real parathyroid
hormone receptor, you die as a baby of a severe
dyschondroplasia. Carriers are asymptomatic.
THYMUS
Originates from the third and (* ?) fourth pharyngeal pouches (* hence, a good location for an
ectopic parathyroid gland.)
Large in neonates, it starts to involute after puberty, and is usually just a mass of fat in older people.
The cortex (many lymphocytes) and medulla (few lymphocytes) have as their basic structural unit
an unusual stellate epithelial cell.
* A few of these cells differentiate as keratinizing squamous pearls, or Hassall's corpuscles.
You are already familiar with the severe underdevelopment of the gland in such
illnesses as ataxia-telangiectasia, DiGeorge / Nezelof, and some (not all) forms
of severe combined immunodeficiency. Usually these people lack good corticomedullary differentiation
and Hassall's corpuscles, as well as lymphocytes.
Thymic cysts are fairly common (3rd branchial pouch), but don't assume all cysts are benign --
for some reason, cysts often form near a thymic cancer (Hodgkin's, germinoma).
* Ask a pathologist about the "myoid cells" in the medulla. They contain
actin, myosin, and myoglobin. Maybe this has something to do with the whole
myasthenia gravis connection.
{14760} normal kid's thymus; a=cortex, b=medulla, c=vessel
* Stress lesions in the thymus are of interest to pathologists, and help
us tell how long somebody was seriously sick. Before 12 hours, there are no changes.
At 12-24 hours, you start seeing macrophages eating lymphocytes.
At 24-48 hours, you start seeing a starry sky. Over 48 hours, the corticomedullary
junction becomes blurry as the gland atrophies. After 72 hours, the gland is atrophic
and there will be no further changes.
Follicular hyperplasia is said to present when there are large germinal follicles in the organ.
Most folks do have a few tiny germinal follicles in the thymus gland. I'd ask you to
reserve the term thymic follicular hyperplasia for situations in which the gland itself is oversized.
This is common in folks with
various autoimmune diseases (especially lupus and addisonism), and in early HIV infection.
Patients with myasthenia gravis, an autoimmune diseases caused by anti-NMJ antibodies, also show
thymic hyperplasia (unless it is removed or destroyed by a thymoma), and thymectomy is the best
treatment for myasthenia gravis. * The less-common form of myasthenia gravis, caused by antibodies
against muscle-specific kinase, is not helped by thymectomy (Brain 126: 2304, 2003).
* True hyperplasia of the thymus is simply a gland that's oversized for the
person's age. For some reason that no one understands, this is fairly common
in adults who have been cured of a malignancy by chemotherapy.
Thymoma is a histologically-benign tumor of the epithelial cells of the thymus gland. There are often lymphocytes mixed in,
but these are non-neoplastic.
The gross appearance (i.e., whether or not the tumor invades the mediastinal structures) is most
important for prognosis.
The World Health Organization (1999) classification of thymomas is now standard,
and predicts outcome quite well (Ann. Thor. Surg. 77: 183, 2004).
Fibrosing variant: Am. J. Clin. Path. 121: 867, 2004.
{13952} thymoma, gross
Among thymoma patients...
Myasthenia gravis occurs in around 50% (* and around 30% of myasthenia gravis patients have a
thymoma; thymomas express acetyl choline receptor epitope on their cells Lancet 339: 707, 1992)
Acquired "pure red cell aplasia" (stop putting out red cells, presumably autoimmune) occurs in
around * 20%
Hypogammaglobulinemia occurs in a few percent and is called Good's syndrome (rare, but
in the "diff" of nearly everything)
* Thymoma series from M.D. Anderson
Cancer 73: 2491, 1994 (not surprisingly, they're for chemotherapy....)
Other thymus tumors:
Lymphomas (T-cell, of course), carcinoids (aggressive, and likely to produce
ACTH or other troublesome hormones: Am. J. Clin. Path. 114: 200, 2000;
Ann. Thor. Surg. 74: 133, 2003; the histology
does not predict behavior Chest 124: 141, 2003),
* carcinomas (i.e., obviously
malignant on histopathology: Cancer 67: 1025, 1991), and * germinomas
(seminomas, less often embryonal-cell carcinomas or choriocarcinomas) arise in the thymus.
* Thymic teratomas are relatively common.
* There's a popular mnemonic for anterior mediastinal masses:
For some reason, there are famous as "the four T's", since one or another always
gets omitted.
* Brian Piccolo, of "Brian's Song" fame, had a thymic embryonal-cell type carcinoma,
as did football player Dan Turk.
* "Status thymicolymphaticus" was an imaginary disease, dreamed up to explain
SIDS and infanticide, for which several million newborns
received radiation therapy. They are now at much increased risk for papillary carcinoma of the
thyroid. (There's a lesson here -- self-deception is certain in the absence of proper controls.)
PINEAL ("the third eye"): Tumors of the pineal are troublesome because of their location. In
children, pineal tumors are likely to produce sexual precocity.
Pinealoma, except for its location, looks exactly like a seminoma or dysgerminoma. Other germ-cell
tumors occur in the pineal also, so you could see a pineal teratoma, a pineal choriocarcinoma, or
any
other. (I've seen all three.)
Pinealoblastoma (see above) resemble neuroblastomas and medulloblastomas.
Pineocytomas are made up of cells like those in adult pineal.
Both are aggressive cancers seen most often in children.
Gliomas, etc., can occur in the pineal. Pineal tumors: Cancer 72: 870, 1993. Cysts: Neurology 41:
1034, 1991 (fooled me once).
{03998} normal pineal gland, anatomy
* THE MELATONIN BUSINESS: Review Am. Fam. Phys. 57(8): 1783, 1998.
Since the stuff is dirt-cheap to make, occurs naturally,
and isn't patentable, it's now widely available over the counter.
If you believed everything you heard about melatonin, you'd be
reading uncritically. What is clear is that its effects on insomnia
and jetlag have impressed people far more than most "supplement" stuff.
Since the pineal often calcifies in older folks, and perhaps fails as
a result, look forward to melatonin as a sleep aid especially in geriatrics.
Expect in the next few years: Studies of the effect of melatonin in (1) seasonal affective disorder; (2)
schizophrenia; (3) obsessive-compulsive; (4) narcolepsy; (5) bipolar disorder; (6) neurodegenerative
diseases; (7) attention deficit disorder; (8) fibromyalgia. I'll predict strong favorable results in at least
one of these categories. In the meantime, the stuff seems extremely safe, and I wouldn't fault you
for wanting to experiment, assuming you give proper informed consent.
 TB of the adrenal
TB of the adrenal
WebPath Photo
 Jack Kennedy, second from left
Jack Kennedy, second from left
PT109 reunion, 1944
Notice weight and pigmentation
{24607} adrenal amyloidosis, gross
{15960} cytomegalic inclusion disease, adrenal
{37216} adrenal leukodystrophy ("Lorenzo's oil") case, gross brain
{37218} adrenal leukodystrophy case, gross brain
{37221} adrenal leukodystrophy case, histology brain
{37224} adrenal leukodystrophy case, gross adrenal
{37225} adrenal leukodystrophy case, histology adrenal
* Some women feel better and have better sexuality if they get some DHEA.
Makes sense. Why? (NEJM 341: 1013, 1999).
{09372} Addison's disease, face
{09373} Addison's disease, buccal pigmentation
{49438} Addison's disease, pigmentation
{49439} Addison's disease, pigmentation
{49440} Addison's disease, atrophy of the adrenal gland
* Expect the unexpected. A lady dies because toluene inhalation
somehow produces adrenal necrosis: J. Tox. 36: 365, 1998.
{09224} adrenal hemorrhage, consistent with Waterhouse Friderichsen
{07570} adrenal hemorrhage, gross, consistent with Waterhouse-Friderichsen syndrome
Etiologies,
from most to least common
Future pathologists: Don't get excited over a lumpy adrenal. Many
people have
a
few adrenal nodules, including a few pigmented ones.
{09370} Cushingism, face
{16109} Cushing's syndrome
{16110} Cushing's syndrome
{16112} Cushing's syndrome "before"
{16111} Cushing's syndrome "after"
{49426} Cushingism, 40 y/o patient
{49427} Cushingism
{49428} Cushingism, hyperplastic adrenal cortex
 Low potassium is likely to cause muscle weakness, and even paralysis.
Low potassium is likely to cause muscle weakness, and even paralysis.

 {49437} adrenogenital syndrome 2 year old girl
{49437} adrenogenital syndrome 2 year old girl
{24450} adrenogenital syndrome, virilized baby girl
{49432} 11-hydroxylase deficiency, 11 month old boy
* It's probably smart to screen for Cushingism. Maybe 20% of these
really are active, and contribute to hypertension, diabetes, obesity,
and osteoorosis, even if the patient is not floridly Cushingoid.
Common sense triumphant. See Surg. Clin. N.A. 84: 875, 2004.
"Occult/subclinical Cushingism due to incidentaloma" is now a recongnized
entity: J. Clin. Endo. Metab. 88: 5808, 2003.
NIH Consensus Conference: Ann. Int. Med. 138: 424, 2003.
{20312} adrenal cortical adenoma, gross
{49436} adrenal cortical adenoma, gross; this one produced Conn's syndrome
{10298} adrenal cortical adenoma
{20315} adrenal cortical adenoma, histology
{09221} adrenal cortical adenoma, histology
{09222} adrenal cortical adenoma, histology
{08964} adrenal cortical adenoma, histology (hard to tell from normal cortex)
{09052} adrenal cortical adenoma, electron micrograph; note tubular cristae in mitochondria
(spaghetti instead of lasagna)
{09375} effect of masculinizing adrenal cortical adenoma, "before"
{09374} effect of masculinizing adrenal cortical adenoma, "after"
{49434} gynecomastia in five-year old boy, feminizing adrenal cortical adenoma
 Adrenal cortical adenoma
Adrenal cortical adenoma
This was a cushingoma
WebPath Photo
{49443} adrenal myelolipoma, gross
{25413} adrenal myelolipoma, histology
 Adrenal myelolipoma
Adrenal myelolipoma
Pittsburgh Pathology Cases
* Another tipoff is broad fibrous bands, but you usually
won't need this to know you're looking at cancer.
{40196} adrenal cortical carcinoma
{24090} adrenal cortical carcinoma, histology
 Metastatic cancer in the adrenals
Metastatic cancer in the adrenals
WebPath Photo
* A large minority of people with non-familial, non-syndromic pheochromocytoma
have a copy of one of these anti-oncogenes (except NF-I) deleted in their non-tumor tissues:
NEJM 346: 1459, 2002. No surprise.
* Bronchial and thymic carcinoids too... the latter mostly in middle-aged men who smoke
a lot. Medicine 76: 21, 1997.
Patients with MEN II may first have adrenal medullary hyperplasia,
which can be nodular. * Nodules over 1 cm are considered pheos.
Using 131-I-MIBG for metastatic malignant pheochromocytoma, with
very good results: Cancer 98: 239, 2003; J. Clin. Endo. Metab. 90:
5888, 2005.
{25417} pheochromocytoma, gross
{49444} pheochromocytoma, gross
{09226} pheochromocytoma, gross
{09227} pheochromocytoma, showing positive brown staining with chromic acid ("chromaffin")
{08874} pheochromocytoma, histology
{08873} pheochromocytoma, histology
{25418} pheochromocytoma, histology
{09228} pheochromocytoma, histology
{09229} pheochromocytoma, histology, positive chromaffin reaction
{09080} pheochromocytoma, electron micrograph showing granules
{09081} pheochromocytoma, electron micrograph showing granules
{08056} pheochromocytoma cardiotoxicity
{08053} pheochromocytoma cardiotoxicity; note contraction bands
<
{25420} neuroblastoma, gross
{25422} neuroblastoma, histology
{39049} neuroblastoma, gross; probably an incidental finding in a newborn
{09009} neuroblastoma, histology
{09232} neuroblastoma, histology
{20046} neuroblastoma, histology
{20047} neuroblastoma, histology
{09011} neuroblastoma, histology, good rosettes
{08963} neuroblastoma histology (sorry, no good rosettes)
{25424} ganglioneuroblastoma, histology
{25426} ganglioneuroblastoma, histology
{24608} ganglioneuroma, gross
 Presacral neuroblastoma
Presacral neuroblastoma
Virtual Hospital
 Neuroblastoma
Neuroblastoma
Electron micrographs
VCU Pathology
* Stay tuned for monoclonal antibodies as a mainstay in the treatment
of difficult neuroblastoma patients: Heme-Onc. Clin. N.A. 15: 853, 2001.
 "Primitive neuroectodermal tumor"
"Primitive neuroectodermal tumor"
This one's in the kidney
Loyola Med
PARATHYROID GLANDS: ANATOMY AND PHYSIOLOGY
 Parathyroid Exhibit
Parathyroid Exhibit
Virtual Pathology Museum
University of Connecticut

 Parathyroids
Parathyroids
"Pathology Outlines"
Nat Pernick MD
The N-terminal assay measures the
active hormone, though the form measured by the C-terminal portion stays around longer. (Pitfall:
the C-terminal portion is filtered through the kidneys, and is increased when
the kidneys are underfunctioning even if
parathyroid function is normal.)
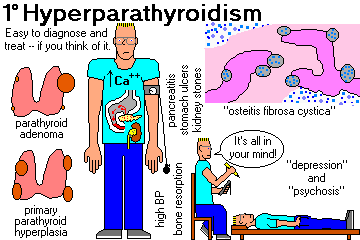
5%... parathyroid carcinoma
15%... parathyroid "hyperplasia" (i.e., all four glands)
 Brown Tumor of Hyperparathyroidism
Brown Tumor of Hyperparathyroidism
Pittsburg Illustrated Case
Occasional overfunctioning do fail to "light up", especially if they are
very small or lack abundant mitochondria (Arch. Oto. 131: 493, 2005).
* Future
pathologists may enjoy reading about the trabecular pattern
that is supposed to be distinctive. Desmoplasia also is a marker
of parathyroid cancer. You are less likely to see obvious
invasion or numerous or weird mitotic figures. As in adrenal
adeonomas, thyroid adenomas, and pheochtomocytomas, hyperchromatic
nuclei ("endocrine atypia") don't mean cancer.
* Watch for the name of "primary parathyroid hyperplasia" to change to
"multiple parathyroid gland neoplasia." The lesion in kidney disease is
a true hyperplasia.
The MEN syndromes do NOT place you at risk
for it. Waterclear hyperplasia is becoming uncommon for some reason.
And you must
have a blood group "O" allele to get it (Hum. Genet. 94: 195, 1994)!!! Gee whiz.
Waterclear adenomas are almost unknown (Arch. Path. Lab. Med. 125: 256, 2002).
{09271} primary parathyroid hyperplasia, histology
 Parathyroid hyperplasia
Parathyroid hyperplasia
You cannot tell this from adenoma by itself
WebPath Photo
This is now routine. You can use the sestamibi scan to see if the graft
is over-functioning: South. Med. U. 93: 215, 2000.
* NOTE: Genetic typing of parathyroid masses has helped us recognize
that the above scheme, while a bit simplistic, is fundamentally accurate.
If one gland is involved, it's an adenoma and will show one of two different genetic
profiles. If two, three, or four glands are involve, it's "multiple gland parathyroid
neoplasia", with different genetic signatures. See Am. J. Path. 165:
565, 2004.
Familial hypocalciuric hypercalcemia, also called "familial benign hypercalcemia", a mild,
autosomal dominant disorder.
Jansen's metaphyseal chondrodysplasia, an autosomal dominant
syndrome caused by an overactive PTH1R parathyroid
hormone receptor. Hypercalcemia and short-limbed dwarfism.
Imprinting: Am. J. Hum. Genet. 68:
1283, 2001. In kidney (PTH-receptor), thyroid (TSH receptor),
ovary (FSH-LH recptors), pituitary (getting worked out), and elsewhere
(growth hormone recetors: J. Clin. Endo. Metab. 88: 4070, 2003);
only Mom's allele
is expressed. In bone, both Mom and Dad's alleles are expressed.
 Child with pseudo-pseudo-hypoparathyroidism
Child with pseudo-pseudo-hypoparathyroidism
Courtesy of Mary Fay MD
 Thymus Exhibit
Thymus Exhibit
Virtual Pathology Museum
University of Connecticut
{13958} Hassall's corpuscles stained for keratin (this appears to be normal thyroid)
* From best-to-worst prognosis, they are:
Type A: Spindle or oval epithelial cells, no atypia, no lymphoytes
A's can expect cures, AB's are usually cured (Ann. Thorac. Surg. 77:
1183, 2004). The rest are more dangerous, but the stage of the tumor at presentation is more important,
and pathologists cannot distinguish the entities in type B very well: Chest 127:
755, 2005.
Type AB: Like A, but with some areas rich in lymphocytes
Type B1: Areas that look like normal thymic cortex, and areas that look like normal thymic medulla
Type B2: Plump epithelial cells with vesicular nuclei and big nucleoli; big perivascular spaces; palisades around vessels
Type B3: Rounded / polygonal epithelail cells growing in sheets, only a few lymphocytes
Type C: Obvious atypia, less recognizable as thymus. There are over a dozen variants described.
{25653} thymoma, gross
{13955} thymic tumor, possibly Hodgkin's
{49097} malignant thymoma, gross
 Primary thymic lymphoma
Primary thymic lymphoma
Virginia
Good pictures
 Mediastinal Masses
Mediastinal Masses
Queen's U., Kingston
Thanks Dr. Boag
 Carcinoid
Carcinoid
Mediastinum
Pittsburgh Pathology Cases
{02815} normal pineal gland, gross
{01223} normal pineal gland
{01239} normal pineal gland histology, with brain sand
{05219} pineal cyst, gross
{01711} pineal germinoma, gross
A man said to the universe:
"Sir, I exist!"
"However," replied the universe,
"That fact has not created in me
A sense of obligation."
-- Stephen Crane
| Visitors to www.pathguy.com reset Jan. 30, 2005: |
Ed says, "This world would be a sorry place if
people like me who call ourselves Christians
didn't try to act as good as
other
good people
."
Prayer Request
 Teaching Pathology
Teaching Pathology
PathMax -- Shawn E. Cowper MD's
pathology education links
Ed's Autopsy Page
Notes for Good Lecturers
Small Group Teaching
Socratic
Teaching
Preventing "F"'s
Classroom Control
"I Hate Histology!"
Ed's Physiology Challenge
Pathology Identification
Keys ("Kansas City Field Guide to Pathology")
Ed's Basic Science
Trivia Quiz -- have a chuckle!
Rudolf
Virchow on Pathology Education -- humor
Curriculum Position Paper -- humor
The Pathology Blues
 Ed's Pathology Review for USMLE I
Ed's Pathology Review for USMLE I
 | Pathological Chess |
 |
Taser Video 83.4 MB 7:26 min |