Ed Friedlander, M.D., Pathologist
scalpel_blade@yahoo.com


Cyberfriends: The help you're looking for is probably here.
Welcome to Ed's Pathology Notes, placed here originally for the convenience of medical students at my school. You need to check the accuracy of any information, from any source, against other credible sources. I cannot diagnose or treat over the web, I cannot comment on the health care you have already received, and these notes cannot substitute for your own doctor's care. I am good at helping people find resources and answers. If you need me, send me an E-mail at scalpel_blade@yahoo.com Your confidentiality is completely respected.
 DoctorGeorge.com is a larger, full-time service.
There is also a fee site at myphysicians.com,
and another at www.afraidtoask.com.
DoctorGeorge.com is a larger, full-time service.
There is also a fee site at myphysicians.com,
and another at www.afraidtoask.com.
Translate this page automatically
 |
With one of four large boxes of "Pathguy" replies. |
 I'm still doing my best to answer
everybody.
Sometimes I get backlogged,
sometimes my E-mail crashes, and sometimes my
literature search software crashes. If you've not heard
from me in a week, post me again. I send my most
challenging questions to the medical student pathology
interest group, minus the name, but with your E-mail
where you can receive a reply.
I'm still doing my best to answer
everybody.
Sometimes I get backlogged,
sometimes my E-mail crashes, and sometimes my
literature search software crashes. If you've not heard
from me in a week, post me again. I send my most
challenging questions to the medical student pathology
interest group, minus the name, but with your E-mail
where you can receive a reply.
Numbers in {curly braces} are from the magnificent Slice of Life videodisk. No medical student should be without access to this wonderful resource. Someday you may be able to access these pictures directly from this page.
Also:
Medmark Pathology -- massive listing of pathology sites
Freely have you received, freely give. -- Matthew 10:8. My
site receives an enormous amount of traffic, and I'm
handling about 200 requests for information weekly, all
as a public service.
Pathology's modern founder,
Rudolf
Virchow M.D., left a legacy
of realism and social conscience for the discipline. I am
a mainstream Christian, a man of science, and a proponent of
common sense and common kindness. I am an outspoken enemy
of all the make-believe and bunk which interfere with
peoples' health, reasonable freedom, and happiness. I
talk and write straight, and without apology.
Throughout these notes, I am speaking only
for myself, and not for any employer, organization,
or associate.
Special thanks to my friend and colleague,
Charles Wheeler M.D.,
pathologist and former Kansas City mayor. Thanks also
to the real Patch
Adams M.D., who wrote me encouragement when we were both
beginning our unusual medical careers.
If you're a private individual who's
enjoyed this site, and want to say, "Thank you, Ed!", then
what I'd like best is a contribution to the Episcopalian home for
abandoned, neglected, and abused kids in Nevada:
My home page
Especially if you're looking for
information on a disease with a name
that you know, here are a couple of
great places for you to go right now
and use Medline, which will
allow you to find every relevant
current scientific publication.
You owe it to yourself to learn to
use this invaluable internet resource.
Not only will you find some information
immediately, but you'll have references
to journal articles which you can obtain
by interlibrary loan, plus the names of
the world's foremost experts and their
institutions.
Alternative (complementary) medicine has made real progress since my
generally-unfavorable 1983 review linked below. If you are
interested in complementary medicine, then I would urge you
to visit my new
Alternative Medicine page.
If you are looking for something on complementary
medicine, please go first to
the American
Association of Naturopathic Physicians.
And for your enjoyment... here are some of my old pathology
exams
for medical school undergraduates.
I cannot examine every claim which my correspondents
share with me. Sometimes the independent thinkers
prove to be correct, and paradigms shift as a result.
You also know that extraordinary claims require
extraordinary evidence. When a discovery proves to
square with the observable world, scientists make
reputations by confirming it, and corporations
are soon making profits from it. When a
decades-old claim by a "persecuted genius"
finds no acceptance from mainstream science,
it probably failed some basic experimental tests designed
to eliminate self-deception. If you ask me about
something like this, I will simply invite you to
do some tests yourself, perhaps as a high-school
science project. Who knows? Perhaps
it'll be you who makes the next great discovery!
Our world is full of people who have found peace, fulfillment, and friendship
by suspending their own reasoning and
simply accepting a single authority which seems wise and good.
I've learned that they leave the movements when, and only when, they
discover they have been maliciously deceived.
In the meantime, nothing that I can say or do will
convince such people that I am a decent human being. I no longer
answer my crank mail.
This site is my hobby, and I presently have no sponsor.
This page was last updated February 6, 2006.
During the ten years my site has been online, it's proved to be
one of the most popular of all internet sites for undergraduate
physician and allied-health education. It is so well-known
that I'm not worried about borrowers.
I never refuse requests from colleagues for permission to
adapt or duplicate it for their own courses... and many do.
So, fellow-teachers,
help yourselves. Don't sell it for a profit, don't use it for a bad purpose,
and at some time in your course, mention me as author and KCUMB as my institution. Drop me a note about
your successes. And special
thanks to everyone who's helped and encouraged me, and especially the
people at KCUMB
for making it possible, and my teaching assistants over the years.
Whatever you're looking for on the web, I hope you find it,
here or elsewhere. Health and friendship!
 I am presently adding clickable links to
images in these notes. Let me know about good online
sources in addition to these:
I am presently adding clickable links to
images in these notes. Let me know about good online
sources in addition to these:
Pathology Education Instructional Resource -- U. of Alabama; includes a digital library
Houston Pathology -- loads of great pictures for student doctors
Pathopic -- Swiss site; great resource for the truly hard-core
Syracuse -- pathology cases
Walter Reed -- surgical cases
Alabama's Interactive Pathology Lab
"Companion to Big Robbins" -- very little here yet
Alberta
Pathology Images --hard-core!
Cornell
Image Collection -- great site
Bristol Biomedical
Image Archive
EMBBS Clinical
Photo Library
Chilean Image Bank -- General Pathology -- en Español
Chilean Image Bank -- Systemic Pathology -- en Español
Connecticut
Virtual Pathology Museum
Australian
Interactive Pathology Museum
Semmelweis U.,
Budapest -- enormous pathology photo collection
Iowa Skin
Pathology
Loyola
Dermatology
History of Medicine -- National Library of Medicine
KU
Pathology Home
Page -- friends of mine
The Medical Algorithms Project -- not so much pathology, but worth a visit
National Museum of Health & Medicine -- Armed Forces Institute of Pathology
Telmeds -- brilliant site by the medical students of Panama (Spanish language)
U of
Iowa Dermatology Images
U Wash
Cytogenetics Image Gallery
Urbana
Atlas of Pathology -- great site
Visible
Human Project at NLM
WebPath:
Internet Pathology
Laboratory -- great siteEd Lulo's Pathology Gallery
Bryan Lee's Pathology Museum
Dino Laporte: Pathology Museum
Tom Demark: Pathology Museum
Dan Hammoudi's Site
Claude Roofian's Site
Pathology Handout -- Korean student-generated site; I am pleased to permit their use of my cartoons
Estimating the Time of Death -- computer program right on a webpage
Pathology Field Guide -- recognizing anatomic lesions, no pictures
St.
Jude's Ranch for Children
I've spent time there and they are good. Write "Thanks
Ed" on your check.
PO Box 60100
Boulder City, NV 89006--0100
More of my notes
My medical students
Clinical
Queries -- PubMed from the National Institutes of Health.
Take your questions here first.
HealthWorld
Yahoo! Medline lists other sites which may work well for you

We comply with the
HONcode standard for health trust worthy
information:
verify
here.

What's a 'double-blind study'? Two pathologists trying to read an EKG!
--Anonymous
Once I had brains, and a heart also; so having tried them both, I should much rather have a heart.
--The Tin Woodsman of Oz
Does CPR work better if you do your compressions with a toilet plunger? The great controversy, including a frank admission that CPR....: JAMA 273: 1299, 1995.
|
|
|
|
|
|
QUIZBANK
Heart (all)
|
|
|
|
|
|
|
|
Looking at pictures of the heart? Remember:
 I think that the pathology of the heart presents fewer difficulties than any other organ system except
GI tract, as long as you understand the physiology.
I think that the pathology of the heart presents fewer difficulties than any other organ system except
GI tract, as long as you understand the physiology.
A variety of genetic syndromes produce various problems with the cardiovascular system. I have tried to resist the tremendous temptation to describe all my favorites. Instead, I've included only the ones that a generalist should know.
Define and use the following terms:
Describe the changes in the myocardium in a trained aerobic athlete, and recognize that these are desirable rather than harmful.
Review the general pathology of congestive heart failure. You should probably already know this from your earlier studies of cardiac physiology and the pathology of the body fluids.
Describe the clinical spectrum of atherosclerotic coronary artery disease.
Tell how the various kinds of angina pectoris arise. Explain how myocardial infarcts occur, why they are so serious, and what the pathologist will see at autopsy under varying circumstances. Tell how subendocardial infarcts occur. Describe the typical picture of chronic ischemic cardiac disease. Tell what a pathologist will find in classic coronary "sudden death", and when the diagnosis can and cannot be made.
Mention the other causes of ischemic heart disease, and tell about how they operate. Tell about the other causes of sudden death.
 Define and use the following terms:
Define and use the following terms:
atresia
clubbing
concentric hypertrophy
congenital heart disease
cor triloculare biatriatum
cyanotic congenital heart disease ("blue baby")
dilatation
Eisenmenger's syndrome
endocarditis
hypertensive heart disease
jet lesion
late cyanosis
paradoxical embolus
polycythemia
pressure overload
reperfusion injury
shunt
transposition
Recall the upper limit of normal weight of the sedentary adult's heart, left ventricular thickness, and right ventricular thickness.
List the minimal anatomic criteria for hypertensive heart disease. Describe the role of pressure overload (clear) and chronic catecholamine stimulation (probable) as causes of this hypertrophy. Describe the gross and microscopic changes typical of the hypertensive's heart. Appreciate that this is a very common finding, both clinically and at autopsy. Explain the difficulty of making the diagnosis when left ventricular failure confuses the picture.
Distinguish cor pulmonale from right heart enlargement caused by left ventricular failure or by congenital malformations. Recognize pulmonary embolization as the only setting for true "acute cor pulmonale". Describe the hypoxic vascular response, and describe how the shape of the right ventricle on cross section differs from normal in this setting. Recognize cor pulmonale as a sufficient explanation for sudden death cases coming to autopsy. Appreciate the tremendous clinical importance of cor pulmonale in many settings.
Recall the two principal causes of serious congenital heart disease. Recall the incidence per thousand live births, and the risk of recurrence. List the problems common to all these children, and the hazards presented by jet lesions.
List tetralogy of Fallot (most common), transposition of the great arteries, persistent truncus arteriosus, and tricuspid atresia as the four most important forms of congenital cyanotic heart disease, and clearly explain the abnormal anatomy and physiology of each. Explain the seriousness of the right-to-left shunt, the most dreaded consequences of paradoxical embolization, and other problems faced by these patients.
Diagram tetralogy of Fallot, listing the four features that define the syndrome.
Describe the usual pattern in transposition of the great vessels, and how a septal defect permits survival after birth. Distinguish "corrected transposition".
Define truncus arteriosus, and recall that it leads eventually to pulmonary hypertension.
Recall ventricular septal defect, atrial septal defect, and patent ductus arteriosus as the major causes of congenital left-to-right shunt. Explain the associated hazards, and especially why cyanosis develops weeks to years after birth in patients with left-to-right shunts.
Recall the location of most ventricular defects. Explain the reason that a "VSD" is unwholesome. Give the meaning of "Roger's disease" and the spontaneous closure rate.
Describe the usual clinical course in atrial septal defect, and tell why these are so seldom recognized in youth. Distinguish ostium primum ("endocardial cushion"), ostium secundum, and sinus venosus atrial septal defects. Recognize ostium primum as the usual form in Down's syndrome, as ostium secundum as most common in other people.
Locate the normal ductus arteriosus and define its function and fate. Identify prostaglandin E as maintaining the patency of the normal ductus. Recognize that in congenital heart disease with impaired blood flow to the lungs, it is good for the ductus to remain open.
Describe patent ductus arteriosus, mentioning its relationship to other defects and to Turner's syndrome, and its most common location. Tell why preductal coarctation can cause right sided heart failure in utero. Describe how it causes hypertension, and mention clinical findings that would alert the pediatrician to post-ductal coarctation. Mention the reasons for getting it fixed surgically.
Recognize pulmonary stenosis with intact interventricular septum as a common, serious cardiac malformation.
Describe the problems caused by a bicuspid aortic valve. Describe the aortic valve in congenital valvular aortic stenosis, and the defect in congenital sub-valvular stenosis. Explain why aortic stenosis commonly produces sudden death.
Give a short account of each:
Anitschkow cell / Aschoff body
antihyaluronidase
antistreptolysin O ("ASO")
Barlow's syndrome
caterpillar cell
dextrocardia with situs inversus
dextrocardia, isolated
erythema marginatum
friable
Kartagener's syndrome
lines of closure
MacCallum's patches of the left atrium
mid-systolic click
regurgitation
Roth spots
situs inversus totalis
splinter hemorrhages
Sydenham's chorea ("St. Vitus Dance")
tamponade
valvular insufficiency
valvular stenosis
vegetations
Relate dextrocardia, Kartagener's syndrome, immotile cilia, and situs inversus totalis.
Remember that mitral stenosis is virtually always caused by scarring from rheumatic fever.
List the important causes all eight valvular syndromes.
List the three important causes of acquired aortic valve stenosis. Sketch a normal (three cusp) aortic valve with calcific stenosis, mention the age of onset, and explain why the process is so serious. Sketch a bicuspid valve with the same thing, and mention which kind of valve is more prone to this.
Describe Barlow's "syndrome" of the mitral valve. Tell how prevalent the disease is, describe the relationship to Marfan's syndrome, and tell what makes the mid-systolic "click". Describe the four complications (bacterial endocarditis, mitral insufficiency, rhythm disturbances, and cardiac neurosis) that can result.
Describe the essential pathogenesis of rheumatic fever and rheumatic pancarditis, and the typical time of onset. List the six principal findings, and describe the changing incidence of the disease in the U.S. and globally. Mention the recurrence rates cited after repeat strep throat. Describe a typical Aschoff body, and tell where it is located. Explain why rheumatic endocarditis is considered more serious in the long run than the myocarditis or pericarditis, and describe the locations of the lesions on the valves in the acute illness.
Describe the pathologic anatomy in chronic mitral valve deformity and chronic aortic valve deformity following rheumatic fever.
Explain why infective (i.e., bacterial) endocarditis is so serious. Tell ways in which the blood becomes seeded with microbes, and times and places where the fibrin-platelet thrombi form inside the heart. Describe acute infective endocarditis, the types of valves which may be involved, its usual cause, and the fatality rate. Name the bacterium most often responsible for subacute bacterial endocarditis. Tell what you will see grossly and microscopically. Tell what valve are most often involved in IV drug-users and in other people. Mention why bacterial endocarditis might be "culture negative". Describe the dread complications of bacterial endocarditis in some detail, and mention clues to the diagnosis. Tell what healed bacterial endocarditis looks like.
Describe typical settings for nonbacterial thrombotic endocarditis ("marantic endocarditis"). Describe the gross and microscopic lesions.
Describe calcification of the mitral annulus as seen in some older individuals, and describe its clinical significance.
Describe the gross, microscopic, and functional lesions in carcinoid syndrome, and explain why we think that the lesions usually occur only on the right side.
Recognize the five complications of valve replacement.
Give a short account of each:
adriamycin
cardiomyopathy
Chagas's disease
daunorubicin
doxorubicin
effusion
myocarditis
Distinguish "myocarditis" (i.e., inflammatory, i.e., autoimmune or infection) and "cardiomyopathy" (i.e., a noninflammatory disorder).
Describe the gross and microscopic pathology and clinical course of a typical case of myocarditis. Recall viruses, especially Coxsackie A & B as the most important causes of significant acute myocarditis, and that this is (fortunately) rare. Mention why we think much of the damage is immune-mediated. Explain why we think many cases of "idiopathic dilated cardiomyopathy" ("Barney Clark's disease") result from Coxsackie myocarditis.
Given a cardiomyopathy, subclassify it as dilated ("flabby heart"), hypertrophic ("muscle-bound heart"), or restrictive-infiltrative-obliterative (i.e., amyloid, "stiff heart").
Recall "dilated-congestive" cardiomyopathy as an end-stage of various longstanding cardiac injuries, and describe the way this heart looks and functions. Describe the histology, and why mural thrombi form.
Cite the clinical features of alcoholic cardiomyopathy, and relate it to cobalt toxicity and beriberi. Recognize alcohol itself as a controversial cause of cardiomyopathy.
Mention the typical setting for peripartum cardiomyopathy, and explain why we suspect a nutritional deficiency.
Recall that disarray of the myocardial fiber arrangement as the typical, though not invariable, feature of hypertrophic cardiomyopathy. Recognize "asymmetric septal hypertrophy" and "idiopathic hypertrophic subaortic stenosis" as the classic hypertrophic cardiomyopathy in which the septum is primarily involved. Recognize "obstructive hypertrophic cardiomyopathy" as the feared consequence of an over-thick septum, and describe this syndrome. Cite the gene responsible for many of these cases.
Briefly describe the heart disease seen in sarcoidosis and systemic amyloidosis, and recall the prevalence of minor amyloid deposits in the hearts of the elderly.
Describe endomyocardial fibrosis as seen in the apices of hearts of young Africans. Describe Loeffler's endocarditis ("with eosinophils") clinically and histologically. Describe endocardial fibroelastosis as seen in U.S. infants, both grossly and clinically.
Describe cardiac damage from anthracyclines (adriamycin and its relatives), and from cocaine.
Recall ruptured MI, penetrating injury, and backwards rupture of an aortic dissection as the only common causes of hemopericardium.
Tell how much fluid is required to produce cardiac tamponade, and under what circumstances it must accumulate.
Recognize the causes of pericarditis from table 13-9 of "Big Robbins". Mention the classic posture assumed by patients with pericarditis. Mention some of the organisms (TB, viruses) that may come from a serous pericardial effusion.
Recognize myocardial infarcts, uremia, radiation, lupus, rheumatic fever and trauma as the causes of fibrinous pericarditis, describe the origin of the distinctive physical sign, give the gourmet comparisons, and mention the anatomic progression and clinical prognosis.
Describe the causes and outcome of purulent (suppurative) pericarditis. List the significant causes of hemorrhagic pericarditis (i.e., TB and cancer) and caseous pericarditis (TB).
Recognize the cancers that tend to metastasize to heart. Be aware of the problems that such metastases can cause, and the difficulty of making the diagnosis.
Recall atrial myxomas ("wrecking balls") as the only common primary tumors of the heart. Tell where they arise and how they cause problems. Recognize their gross and microscopic appearances.
Recognize any good example of each of the types of lesions depicted in the videodisc series.
Say "REE-nin", not "RENN-in", when talking about that important hormone from human physiology. Rennin is from a calf's stomach and you use it to make cheese.
The heart has its reasons of which Reason knows nothing. -- Pascal
INTRODUCTION
Cardiac pathology is relatively straightforward, if you understand the heart's physiology.
There are only a few important diseases and patterns of injury, and most of these are fairly well understood.
 * THE PROARRHYTHMIAS FIASCO
* THE PROARRHYTHMIAS FIASCO
Had CAST not included a placebo group, the erroneous conclusion that drug therapy did no harm might have been reached.
--NEJM, cited above.
"Proarrhythmias" are rhythm disturbances generated or made worse by anti-arrhythmic drugs. And they are quite common. In past decades, there have been fads for prescribing anti-arrhythmic drugs to asymptomatic people with ordinary ventricular ectopic beats (PVC's) and who have never had a heart attack. This is indefensible (Am. J. Card. 64: 50-J, 1989; NEJM 312: 193, 1985), and we can only guess how many thousands of people have died worldwide as a result.
ATHLETE'S HEART (is good: Eur. Heart. J. 17: 127, 1996).
The heart is special, because one of the most common "abnormalities" is the desirable result of
vigorous aerobic training.
Further, the athlete develops tremendous collateral circulation. If something happens to one
coronary artery, the percentage of lost myocardium (if any), and the absolute mass of lost
myocardium, are likely to be less. Surviving a major rhythm disturbance may still be a problem, but
death from cardiogenic shock in the acute phase is unlikely.
Also, exercise does offer some protection from coronary artery atherosclerosis. Most runners also
avoid tobacco and cholesterol-raising foods, and exercise tends to keep hypertension
and adult-onset
diabetes at bay, making it harder to sort out the benefit of exercise itself.
Nevertheless, even the best runners enjoy no
absolute immunity to coronary artery atherosclerosis, with all its
serious side-effects.
People who weren't thinking (including some physicians in the 1950's) used to talk about "athlete's heart syndrome" as
if it were something to be avoided. The "reasoning" was that failing hearts in disease tend to be
hypertrophied, and.... Silly, okay. Probably the only common situation in which a person
should avoid heavy aerobic training is some birth defects in which cardiac hypertrophy is likely to
impair outflow from the left ventricle. (Outstanding among these is hypertrophic cardiomyopathy).
* One group reports that athletes' hearts do not hypertrophy to a thickness
of more than 12 mm unless their chambers are also dilated, which should help
make the distinction from diseased hearts: J. Am. Coll. Card. 40: 1431, 2002;
this surprises me.
CONGESTIVE HEART FAILURE ("CHF"; review Am. Heart J.
138: 5, 1999)
Inability of the heart to handle the volume of blood returned to it.
Either the heart muscle cannot pump because of intrinsic disease, or the blood is flowing in the
wrong way, or the heart must pump against excessive resistance, or the heart must pump a
preposterously large amount of blood (the latter is "high output failure").
Physiologists speak of "forward failure" (i.e., inability to perfuse the arteries) and "backward
failure" (i.e., congestion and its problems). Both occur simultaneously, of course, but one or the
other may be more obvious clinically.
The distinction between "congestive heart failure" and "cardiogenic shock" is admittedly artificial.
"Cardiogenic shock" is a term reserved for the acute situation (usually a myocardial infarct);
"failure" can simply mean inability to handle the ordinary venous return.
Exactly why the over-burdened heart's strength starts to fail is often unclear, and its response to
pharmacologic interventions often makes the picture more mysterious. There is talk of induction of
an abnormal myosin isoenzyme which is a poor ATP-ase, as well as decreased numbers of beta
sympathetic receptors, etc.
There's much interest in the effects of heart failure itself on the heart, both changes in the shape of
the heart that render its pumping and/or filling less effective ("remodelling"; Am. Heart J. 130: 153, 1995), and
problems with the cells themselves (notably failure of reuptake of calcium from the sarcoplasmic
reticulum; see Am. Heart J. 129: 684, 1995).
* Why is the heart of a fat person bigger? Is it simply from
the extra exercise of carrying around 100-200 or more pounds of weight?
Or is it the result of lack of adiponectin secretion by overstuffed
adipocytes (Nat. Med. 10: 1384, 2005)? How can anybody tell?
Tumor necrosis factor is one of the "usual suspects" here as well,
and there are some efforts to help CHF by administering its receptor
("etanercept": Circulation 99: 3213, 1999).
You can help out a congestive-heart-failure person, somewhat, by improving aerobic muscle tone
(i.e., more efficient burning of fuel), but exercise is no panacea (J. Am. Coll. Card. 25: 1239, 1995;
J. Am. Coll. Card. 27: 140, 1996; JAMA 283: 3095, 2000).
* Future pathologists: Serum cardiac troponin T (already in use
pre-hospital to screen for MI's: Am. Heart J. 138: 45, 1999)
as a marker for how
bad my congestive heart failure is today: Am. Heart J 138: 95, 1999.
Nesiritide, a natriuretic peptide originally found in brain,
helps CHF: NEJM 343: 246, 2000; you can also measure levels
to detect CHF (NEJM 347: 161, 2002; B-type natriuretic peptide
triumphs as a way to distinguish CHF from other causes of dyspnea:
NEJM 350: 647, 2004). No surprise.
You'll need to know this artificial classification of
cardiac hypertrophies, since
a clinician or radiologist may ask you about it.
Eccentric hypertrophy: The heart is not able
to empty properly, because it's pumping too much blood
(anemia, AV shunts, thyroid disease, others) and/or
it refills (aortic regurgitation) and/or it's
doing its best but can't keep up for whatever reason
(i.e., congestive heart failure from most causes).
Thick wall, chamber is very expanded and does not empty adequately.
Looks bigger than a concentrically hypertrophied heart of the same weight
on a chest x-ray. (Why?)
Physiologic hypertrophy: Aerobic athlete.
Thick wall, the chamber can fill tremendously but empties very well.
Hypertrophic cardiomyopathy: Uneven fiber
enlargement and scrambling
not to be confused with any of the above. Bumps
on the heart muscle notably around the aortic outflow
track.
Measurements for future pathologists:
350 gm...
Normal upper limit of weight for an adult couch potato's heart
1.5 cm...
Normal upper limit of thickness for an adult couch potato's left ventricle
0.5 cm...
Normal upper limit of thickness for an adult couch potato's right ventricle
Measurements don't include the trabeculae carnae.
* Fun to know: If the heart was once very hypertrophic but is so no longer
(i.e., an athlete gone to seed, a hypertensive or valve-disease patient
successfully treated), the anterior and posterior descending coronaries are
very wiggly. Why?
Left-sided congestive heart failure
Failure of the left side of the heart to pump sufficient blood.
Except in the case of pure mitral stenosis (why?) or amyloidosis (why?), the left ventricle will be
hypertrophied and dilated. The left atrium will usually be, also (and especially in mitral valve
disease, why?)
The common causes of left-sided failure
Ischemia (old or recent myocardial infarct, ischemic muscle disease)
Aortic or mitral valve disease
Systemic hypertension
Myocardial disease / cardiomyopathy
NOTE: Of these, uncontrolled "systemic hypertension" (i.e., too much blood to push through too-narrow arterioles) is the
most common; when the heart fails, blood pressure drops, making the true cause
less obvious. See JAMA 273: 1363, 1996 (Framingham). How it progresses: JAMA 275: 1557,
1996; J. Am. Coll. Card. 25: 888, 1995.
The common effects of left-sided failure
First, on exertion
Later, paroxysmal nocturnal dyspnea ("cardiac dyspnea"); on lying down for a while, fluid
redistributes itself in the body, resulting in pulmonary edema. I think that the
reason that it's paroxysmal (i.e., comes on all of a sudden)
is that as the lungs become heavier (i.e., congestion, maybe edema)
their weight presses on the pulmonary veins which in turn
makes them more congested. Patients may throw the windows open
at night, or learn to sleep on various numbers of pillows; you the physician will hear rales; the
pathologist may see "brown induration" and hemosiderin-laden "heart failure" macrophages;
remember these?
Diastolic heart failure is a special situation in which the ejection
fraction is normal but the person is still in failure. The ventricle will not relax / is too
stiff to fill properly. It is not rare; the pathophysiology is being worked out
(NEJM 350: 1953, 2004).
High-output failure is a special situation, glossed-over by "Big Robbins", in which the heart fails
because it must pump an excessive among of blood. You'll see dependent edema probably
because the veins of the body constrict extra-hard to return blood to the heart.
The causes:
Anemia
Hyperthyroidism
High fever
Shunts between an artery and a vein
Beriberi (arterioles open)
Paget's disease of bone (abnormal bone vasculature)
Iatrogenic (i.e., shunts in dialysis)
Right-sided congestive heart failure
Failure of the right side of the heart to pump enough blood.
As you'd expect, the right ventricle and atrium will usually be hypertrophied and dilated.
The common causes of right-sided failure
Pulmonary emboli (acute or chronic)
Any disease interfering seriously with lung ventilation
Emphysema
Cystic fibrosis
Fibrosing lung
Most others
NOTE: The mechanism, of course, is increased pulmonary vascular resistance (due to fibrosis
and/or the hypoxic vascular response; remember this?)
Left-sided heart failure!
Cardiac defects with left-to-right shunts (why?)
The effects of right-sided failure
Splanchnic congestion (you'll feel big livers & spleens; check for "hepatojugular reflux")
Jugular venous distention (look carefully)
Total-body dependent edema (from increased venous hydrostatic pressure, etc.)
Effusions (transudates, of course; notably pleural, notably more on the right side than on the left;
why?)
NOTE: "Cardiac cirrhosis" of the liver, often discussed in textbooks as the result of right-sided
failure, almost never happens. The one time you might see it is in longstanding, severe tricuspid
insufficiency, with or without right-sided failure (why?)
NOTE: Some pathophysiologists include cardiac tamponade as a type of right-sided failure.
* Good news: In contrast to studies of selected patient populations
with various illnesses from decades ago, black and white
people with congestive heart failure seem to get equally good treatment (JAMA 289: 2517, 2003).
ISCHEMIC HEART DISEASE
The cause of around 750,000 deaths annually in the U.S. In 90% or more of the cases, the problem
is coronary artery atherosclerosis (ASCVD).
In the setting of acute ischemia, one common mechanism of death is cardiac rhythm disturbances
("arrhythmias", one of the great misnomers in medicine). Don't worry about the details here; just
remember what you've already learned about (1) ischemia making membranes abnormally
permeable to ions, and (2) action potentials and how they result from altered permeability to ions.
Cigaret smoking is a risk factor for coronary atherosclerosis, and also
sensitizes the myocardium to be susceptible to rhythm disturbances in the setting of ischemia.
It's also worth remembering that coronary arteries usually increase their diameters substantially as
atherosclerosis worsens (study from my old department at Bowman-Gray: JAMA 271: 289,
1994), a phenomenon which saves lots of lives.
Future pathologists: We (unlike angiographers) refer to coronary artery stenosis in terms of
percentage of cross-sectional area occluded. Why?
You know the dominant coronary artery is whichever
supplies the posterior descending coronary
artery.
Angina pectoris: Pain in the chest from coronary insufficiency, in the absence of myocardial
infarction
Regardless of its category, all angina is due to some combination of coronary stenosis (usually
atherosclerotic), coronary spasm (demonstrable on angiogram), thromboxane A2 release and platelet
aggregation, and temporarily increased myocardial work load.
Stable ("classic", "typical", "Heberden") angina generally results from increased work in a patient
with coronary atherosclerosis, and relieved by rest.
Generally, three-vessel disease with >75% stenosis in each of three coronary arteries is sufficient to
cause problems. Of course, finding 90+% stenosis is commonplace in the U.S. Ask patients about
exacerbation of pain on climbing stairs or walking against cold wind.
Unstable ("pre-infarction", "crescendo", "acute coronary insufficiency") angina
In most cases, this is probably due to a thrombus developing, by fits and starts (white regions,
organization, etc.), over a ruptured plaque. Untreated, many of these people get an MI soon.
* "Is it really a heart attack?" Sometimes it's tough to know, especially
without an autopsy. "Infarctlets" / "CK leaks" / "troponin-positive acute coronary syndrome"
are now discussed as being coronary ischemic events which raise
cardiac enzymes but do not produce the EKG changes of a "true MI".
No one knows exactly what to do with these patients (Br. Med. J. 324: 377, 2002; the traditional
rx of calcium channel blockers fails more often than not Chest 123: 380, 2003).
* The endothelial nitric oxide synthetase gene has a mutant allele
which is a strong predictor for coronary artery spasm: Circulation 99:
2864, 1999.
* A "mouse model" (?) for Prinzmetal's has a mutation in a minor
potassium pump; sudden cardiac death and coronaries that over-react to
minor vasoconstrictors characterize this mouse: Nat. Med. 8:
466, 2002; J. Clin. Invest. 110: 203, 2002.
Cardiac syndrome X ("microvascular angina"), with classical clinical
angina and wide-open coronary arteries, and a generally good prognosis,
is an autonomic (?) disturbance in which the smooth
muscle of blood vessels does not dilate appropriately and/or constricts
too easily. These people may not get the red flush on re-perfusing a forearm made
ischemic by a blood pressure cuff. More about this arcane syndrome,
which is quite common and seems to have something to do with insulin
resistance, in Lancet 342: 136, 1992, and
Am. J. Card. 79: 961, 1997; NEJM 346: 1948, 2002; Hosp. Pract. 35(2):
75, Feb 15, 2000.
* You can diagnose microvascular angina in the lab by injecting acetylcholine.
Microvascular angina update: Lancet 351: 1165, 1998.
Don't confuse it with metabolic syndrome X, which is the poorly-understood, all-too-common
syndrome of obesity, hypertriglyceridemia, low HDL,
hypertension, and insulin resistance.
Myocardial infarction: Death of a portion of myocardium due to loss of the blood supply.
This common (maybe 1 million/year in the U.S.) catastrophe underlies many, but by no means all,
fatal cases of ischemic heart disease.
There are fewer myocardial infarcts nowadays than in the past, and the odds for a patient with a
myocardial infarct are much better, too, especially once he or she has made it to the hospital (NEJM
334: 884, 1996). Causes of myocardial infarcts
Atherosclerosis: Makes up 90% of coronary artery disease
{03476} atherosclerosis, coronary artery
The pathologist can usually find either a ruptured plaque (often with an overlying thrombus, hence
the archaic name "coronary thrombosis"; review of coronary thrombi Am. J. Card. 68: 28B, 1991),
or (less often) a hemorrhage into a plaque, ballooning its cap against the opposite wall. If neither are
present, but there's horrendous atherosclerosis and no other explanation, we assume the thrombus
lysed.
No surprise: The Armed Forces Institute of Pathology
documents
that sudden cardiac death occurring during intense
physical exertion typically results from a ruptured plaque:
JAMA 281: 921, 1999.
Long afterwards, look for a recanalized thrombus.
*Space-age medicine! Viewing the thrombus by angioscopy NEJM 326: 287, 1993. Unstable
angina thrombi are likely to be white fibrin-platelet thrombi or organizing thrombi (why?), etc. And
the thrombi overlie plaques which are lipid-rich and/or disrupted (no surprise): Am. J. Card. 79:
1106, 1997.
Cocaine: Rough on the heart, and (your lecturer believes) the second most common cause of myocardial
infarction and sudden cardiac death in the U.S. Review Med. Clin. N.A. 89:
1323, 2005; South. Med. J. 98: 794, 2005.
The recreational drug (1) produces coronary artery constriction (spasm, or whatever, nobody really
understands it NEJM 333: 1267, 1995; Am. J. Card. 79: 492, 1997) and cardiac ischemia
and even infarction (Circulation 99: 2737, 1999),
especially when combined with cigaret smoking (NEJM 330: 454, 1994), which is bad because both
increase the heart's need for oxygen; (2) makes the heart more prone to rhythm disturbances, perhaps
by enhancing the effects of endogenous catecholamines; (3) can produce single-fiber necrosis and
contraction bands (something to do with ion channels), perhaps leading to myocarditis and/or dilated
cardiomyopathy.
Future pharmacologists: The drug opens sodium channels, perhaps opens calcium channels, and
prevents synaptic re-uptake of catecholamines. Crystal meth probably does
the same thing (J. Tox. 41: 981, 2003).
* Hopefully no one was surprised by the results of a huge study
that showed that cocaine's effects on the heart are not mediated by its
causing precocious atherosclerosis (Am. Heart. J. 150: 921, 2005).
Prinzmetal's coronary spasm
Myocardial bridge and diving coronary artery, especially involving a portion of the left
anterior coronary artery. Vasculitis
Remember (1) lupus; (2) polyarteritis nodosa; (3) rheumatoid arthritis; (4) Kawasaki's;
(5) Takayasu's; (6) mycotic aneurysms (remember what those are? seen in bacterial endocarditis);
(7) rarely, exotic infections.
{06569} polyarteritis nodosa of a coronary artery
Embolization (i.e., bacterial endocarditis)
Syphilis (classic!)
{03525} syphilis. Nice plasma cells.
Dissecting hematoma
A "dissecting aneurysm" can slide right up through a coronary ostium and cause occlusion. Or
trauma can induce this change in an artery.
{06575} coronary artery dissection
Shock and left-sided failure, even if mild, in the setting of subtotal coronary occlusion.
A drop in blood pressure from any cause is likely to produce a subendocardial infarct.
(By definition, a subendocardial infarct is less than 50% as thick as the wall).
This is a
watershed infarct of the myocardium farthest from the coronary, but not close enough to the
chamber to get its nutrients from the blood in the chamber.) If all three coronaries are stenotic, the
infarct is likely to be circumferential.
* A favorite place to find subendocardial infarcts is in the tips
of the mitral valve's papillary muscles. Why?
*Amyloid of the coronaries shouldn't cause an infarct, as the lumen is open
and the endothelium undamaged.
{03386} coronary artery amyloidosis
Clinical picture of the "MI" patient
Everyone knows the "typical" uncomplicated heart attack victim. There is chest pain (maybe,
perhaps "crushing") radiating to the (left arm? jaw? abdomen? wherever?), perhaps with
diaphoresis, perhaps with shortness of breath, perhaps with a feeling of fear, or perhaps with none of
these ("silent infarct").
Serum enzymes (troponin, CK, perhaps with an "MB" cardiac isoenzyme band) begin to rise in perhaps 2-4
hours, but may be normal in a day or two. Ask your cardiologist how often to check. Later, look
for the famous "LDH1>LDH2" isoenzyme flip.
The EKG changes depend on the location. Remember the subendocardial infarcts are notoriously
hard to pinpoint by EKG, and that posterior wall infarcts are easy to miss, too.
You'll learn of the management and treatment of these patients while in the "unit". Remember that
education about reducing risk factors is an important part of cardiac rehabilitation.
*Speaking of "cardiac rehabilitation": As a med student, I watched heart-attack survivors participate
at great expense in kindergarten-level exercise programs in special hospital areas, supervised by
boarded cardiologists. Not surprisingly, this is being replaced (under the much-maligned "managed
care") by expenditures on prevention (Am. J. Card. 79: 1075, 1997); for the "medically indigent"
who need to change their lifestyles, good results are obtained simply by talking and explaining
nicely (Am. J. Card. 79: 281, 1997). Pathology
0-30 minutes Wavy fibers at the edges, loss of glycogen from cytoplasm.
1- 2 hours Mitochondrial calcium, maybe contraction bands, maybe hydropic changes, maybe even a little fatty
change.
4-8 hours Earliest nuclear changes, polys appear; you may see a bit of dark mottling grossly
8-24 hours First clear gross changes, i.e., pallor; good coagulation necrosis; often good contraction bands;
definitely feels soft by 24 hours
24-72 hours Looks terrible, lots of polys, fibers very dead; infarct feels soft and looks pale and yellowish (why?)
3- 7 days Macrophages, granulation tissue starts at rim; grossly you see the red granulation tissue around the
infarct
10 days Nice granulation tissue; macrophage cleanup team may be removing the dead fibers, or the dead fibers
may persist for
weeks
7 weeks Nice scar.
* Today's standard for autopsy reports: "Acute" means polys, "Healing" means
the polys are gone but there are monocytes, "Healed" means the monocytes are gone.
"Microscopic" means less than 10% of the left ventricle (sic.), medium is 10% to 30%,
large is more than 30% (Am. Heart. J. 144: 957, 2001).
Among these, the only items which may be unfamiliar are wavy fibers (they had stopped beating and
were roughed-up by the beating of the rest of the heart) and contraction bands ("myofibrillar
degeneration"; densely eosinophilic cross-bands which probably result from calcium entering
membrane-damaged cells during reperfusion, i.e., reperfusion injury. Remember that?)
*The AFIP has finally documented what real-world pathologists have known and used for decades:
contraction bands let you know that a sudden death is of cardiac ischemic origin (gee whiz, Lancet 347:
1710, 1996)
* Future pathologists also note: Contraction bands can probably result from
epinephrine administration and/or electric shocks in CPR.
NOTE: Classically, the coronary arteries have the following distribution, and their occlusion will
result in transmural (across-the-wall, or
at least more than 50%) infarcts in the corresponding distribution
Right: Posterior-inferior wall, posterior 1/3 of septum
Left anterior descending: Anterior wall, anterior 2/3 of septum
Left circumflex: Lateral wall
There's plenty of variability. Especially when there's atherosclerosis, collateral formation may result
in the "best" artery supplying most of the heart, with minor occlusions producing "infarction at a
distance". Don't worry yet about "which gives you a bundle branch block", etc., etc.
NOTE: Infarcts almost never involve the right ventricle, unless it is extremely hypertrophied (why
do you think?) If the infarct extends as a result of more mayhem in the coronaries, expect a mix of
ages.
*Future pathologists: Try the nitroblue tetrazolium technique to demonstrate early myocardial
infarcts. Drop a slice of heart in the solution, and viable heart, containing an oxidizing enzyme, will
stain brown, and dead heart remain pale. I could never get this to work.
*Future pathologists: Don't mistake livor mortis for a posterior
wall MI!
{10103} myocardial infarct, acute
Complications occur in many but not all myocardial infarcts.
Rhythm disturbances may begin at any time until the damage to the conduction system is healed.
Formerly the great killer of "MI" patients, pharmacologic therapy is now generally successful in
managing these.
Left-sided congestive heart failure results from extensive damage to the heart. Whether or not there's
been a known episode of infarction, a person with severe coronary disease can get intractable heart
failure on the basis of ischemic scarring.
Cardiogenic shock results from necrosis of more than >40% of a non-athlete's myocardium. This is
usually fatal.
Rupture (ouch!) of the heart may occur, typically when the damaged heart is most soft (days 4-10).
Rupture of the free wall will result in hemopericardium, tamponade, and instant death.
Rupture of the septum will result in a sudden left-to-right shunt.
Rupture of the papillary muscle produces severe mitral regurgitation.
{03614} ruptured wall
Aneurysm formation, mural thrombus formation, and embolization are dread, common side-effects
of myocardial infarcts. Ventricular aneurysms begin with the paradoxical movement of the necrotic
myocardium outward during systole; later the fibrous scar balloons. Large infarcts can produce large aneurysms that continue to
balloon out. Having a big aneurysm following a myocardial infarct greatly interferes with pump
effectiveness. Embolization from a mural thrombus (with or without an aneurysm) is often
devastating.
{06323} myocardial ventricular aneurysm
* Dressler's pericarditis / postpericardiotomy syndrome,
is pericarditis (sometimes with life-threatening effusion)
which supposedly
occurs weeks to years after an MI or cardiac surgery.
Dressler's is rare at best (Heart 80: 98, 1998),
and some cardiologists don't believe in it (Angiology 47:
83, 1996).
Chronic ischemic heart disease is cardiac muscle insufficiency
due to scarring from old infarcts, not necessarily large or known to have occurred.
It is a major cause of congestive heart failure. Think of this especially
if your patient has nighttime symptoms but the heart is perhaps not enlarged.
Sudden cardiac death
Definition: Death from cardiac causes in previously-asymptomatic person, within 1 (or 24) hours
after onset of symptoms. Most often, the person feels odd, then falls over dead. Either there's a
rhythm disturbance (most often, and typically "ventricular fibrillation"), or there's some sudden,
severe outflow obstruction. (Of course, the "forme fruste" of sudden cardiac death is a "fainting
spell"!)
Every day, around 1000 people in the U.S. get "sudden cardiac death". (Some of these are probably
included in the "1 million MI's" statistic; some probably aren't.)
Here's a rule: "Sudden death" means "sudden cardiac death". (Of course that includes
pulmonary emboli.) Apart from extreme trauma, severing of a major body vessel,
seizure death,
anaphylactic shock, super-fast poisons, or a hemorrhage that destroys the medulla,
there's probably nothing that can kill a human being in less than an hour that isn't on this list.
The causes of sudden cardiac death
NOTE: Usually 75% stenosis of all 3 coronaries, often more
Being stressed (epinephrine) and having tobacco on board probably exacerbate the rhythm disturbance, in
ischemia due to atherosclerosis or anything else.
* Future whole-person-oriented medical examiners: When somebody drops dead
with no pathology except three narrow coronary arteries, ask "Why today rather than yesterday?"
You will almost always find out if you ask about the circumstances.
Vasospasm
Fiber necrosis
Rhythm disturbances
For example, having only one (Pete Maravich had no left main, also J. For. Sci. 35: 981, 1990), or having one
come off the pulmonary artery. These birth defects can cause other problems, too.
Ask a forensic pathologist about sudden death due to a "diving coronary artery", i.e., one which
enters the myocardium too soon, and "myocardial bridging" (very common in hypertrophic
cardiomyopathy and common enough in "normal people"), in which a band of heart muscle overlies a coronary
artery (NEJM 339: 1201, 1998; Chest 116: 574, 1999).
Atrial myxoma or thrombus obstructing the mitral valve
Hypertrophic cardiomyopathy
Aortic valve stenosis from most any cause
NOTE: This is important. The mechanism is acute coronary insufficiency. (1) The intra-myocardial branches of the coronary
arteries fill only during diastole. In aortic valve stenosis, diastole is
greatly shortened (why?). (2) Bernoulli's principle (remember that?) results in blood being sucked
out of the coronary arteries by the super-fast jet of blood passing through the narrowed aortic valve.
Cor pulmonale
Pulmonary embolus ("Do you think that should count as sudden cardiac death?"; maybe 50,000
extra "sudden death" cases among previously-healthy people in the U.S. per year)
Wolff-Parkinson-White, others ("bypass fibers", bundle of Kent; you'll learn about these on rotations; surgery Sci.
Am. 269(1): 68, July 1993; gene NEJM 344: 1823, 2001)
Amyloid in the bundle of His (real frequency as cause of death is unknown, may be high)
Anti-Ro disease of the unborn and babies ("neonatal lupus")
After surgically-induced trauma
Commotio cordis (myocardial concussion): Piezoelectric effect after a blow to the
chest on top of the T-wave. See
NEJM 333: 382, 1995.
Special hazard for
basketball, baseball catchers,
hockey goalies, other activities: JAMA 287: 1142, 2002.
Glue sniffing sensitizes the heart to rhythm disturbances.
Iron overload (rhythm disturbances)
* Endocardial fibroelastosis (Am. J. For. Med. Path. 20: 357, 1999).
Ventricular septal defect involving bundle of His
* Familial syndromes with apoptosis of the sinus node and AV node
(Circulation 93: 1424, 1996).
Right ventricular dysplasia (see below)
Channelopathies
The unusual EKG is not always expressed, so keep a high index
of suspicion.
Whenever you suspect long-QT, either on EKG or
family history (i.e., unexplained
sudden death, including SIDS, or an episode of
torsade de pointes) or personal history
(torsade, syncope), get consultation; genetic screening for suspected families
will be routine probably by 2005.
This business is very tricky.
You'll learn on rotations what medications are contra-indicated
in which syndromes, when to place a defibrillator, and so forth.
You shouldn't need to be reminded to do an EKG on all pre-sports
physicals where there's a history of syncopal spells or sudden
unexplained death in a family member under age 30.
Swimming seems to trigger sudden death when the mutation is in the
potassium channel KCNQ1 (Mayo Clin. Proc. 74: 1088, 1999),
while loud noises trigger when the mutation is in the potassium channel KCNH2 / HERG.
When the mutation is in the SCN5A channel (same as a Brugada locus
but a different allele), death is likely to occur during sleep.
You also know that prolongation of the QT interval
in response to antipsychotic drugs predicts sudden unexpected
death from drug-induced rhythm disturbance: Lancet 355: 1048, 2000.
Sudden ventricular fibrillation with no anatomic findings
at autopsy.
Previous EKG's showed:
Usually men, more common among people of Asian ancestry, usually
die in their sleep at night.
Runs in families of course, and worth getting an implanted defibrillator for
if you have it (Am. J. Card. 83: 98-D, 1999).
About 3% of Thai and Laotian males have Brugada, and this
accounts for the flap about "delayed death from yellow rain
in Laotian immigrants" in
the 1980's.
The sodium channel mutation (LQT3 / SCN5A,
"idiopathic ventricular fibrillation" --
Nature 392: 293, 1998)
is the Brugada gene.
* Less deadly, but interesting to scientists: KVLQT1 is a gene for
familial atrial fibrillation (Science 299: 251, 2003).
* "Catecholamine-sensitive V-tach" is a childhood disease in which
exercise reproducibly causes the arrhythmias. I bet it is a
channelopathy. It is managed with beta-blockers.
* "Idiopathic ventricular fibrillation", not familial and not caused by
stress, surely covers a few entities which have not yet been discovered.
Update on this and other causes of sudden cardiac death in structurally
normal hearts: J. Am. Coll. Card. 43: 1137, 2004.
NOTE: Syncope can warn of most of these. How?
NOTE: The other major causes of sudden unexpected
death are pulmonary emboli,
anaphylaxis, brain hemorrhages,
and epileptic seizures ("SUDEP"; Lancet 353: 888, 1999; Neurology 57:
430, 2001; future medical
examiners see Am. J. For. Med. Path. 23: 307, 2002).
Other problems in cardiac ischemia
There's no room here to talk about the various rhythm disturbances and kinds of heart block that
may result from coronary insufficiency.
Worth remembering: Atrial fibrillation is a troublesome rhythm disturbance seen in coronary disease,
mitral valve disease (why?), hyperthyroidism (George Bush Sr.; why?),
ectopic (and ablatable) foci in the pulmonary veins
(NEJM 339: 659, 1998), etc. It is especially dangerous
because thrombi tend to form in the quivering atria and embolize; this causes 1/3 of strokes in older
folks. (* Hereditary a-fib: NEJM 336: 905, 1997).
Serious degrees of heart block can cause people with coronary disease (or other problems; remember
amyloid) to drop over suddenly ("Stokes-Adams attacks", etc.) Get them pacemakers.
Despite the slight statistical
advantage of a one-drink-a-day person over a non-drinker with the same other risk factors (Br. Med.
J. 312: 1200, 1996; Br. Med. J. 314: 18, 1997), "your heart" is no reason to drink if you'd prefer not
to. Because of the media hype over red wine ("tannins in wine protect the heart, the French
paradox"), a couple of groups have looked at the cardioprotective properties of how-much vs. what-kind of alcohol (Br. Med. J.
312: 731 & 736, 1996); it's how much alcohol, not the kind of
beverage.
It's now painfully clear that a
person's successful return to near-normal living after a heart attack is largely a function of his or her
knowledge about the disease. Fatalism turns you into a cardiac neurotic, a bad way to live.
Education is the key to success. The implications for a primary-care physician are clear (Br. Med. J.
312: 1191, 1996).
No, it's probably not going to
happen because you were having sex, even if you have angina or had a myocardial infarct (JAMA
275: 1405, 1996).
The Cambridge Heart Anti-Oxidant Study finds a good
protective effect from vitamin E (Lancet 347: 781, 1996).
Hibernating myocardium: The most interesting discovery in "heart
pathology" in several years (NEJM 339, 173, 1998).
Pathologists can spot hibernating cells (at autopsy, of course) by...
*You can usually find a few of these cells normally in the subendocardium.
HYPERTENSIVE HEART DISEASE
In systemic hypertension, the left ventricle undergoes hypertrophy and, later, dilatation.
Probably the hypertrophy is mostly the result of pushing against the greatly increased load (more blood,
more vascular resistance). Some
hypertensives do have abnormally high cardiac outputs, so perhaps in these folks the heart is over-working for its own reasons.
There are rumors that some hypertensives suffer from chronic excess of catecholamines, and perhaps
this exacerbates the hypertrophy.
Ultimately, the hypertensive's left ventricle will probably fail. To make the diagnosis, the heart must
weigh more than 350 gm, and the left ventricle be more than 1.5 cm thick, with no other reason.
You remember the microscopic appearance of the hypertrophied myocardial cell (thick fibers, many-ploid squared "boxcar
nuclei"). It helps if the patient has a history of "high blood pressure", but
when congestive heart failure supervenes on the salt-overloaded, hyper-constricted vascular system,
"the high blood pressure may be cured".
NOTES: Despite "Big Robbins", I doubt that "hypertrophy" itself causes myocyte injury or
heart failure. I think that these result from the underlying hypertension.
NOTE: We talk about the
etiologies of systemic hypertension under "Kidney".
NOTE: Somebody may tell you that most congestive heart failure in the elderly is due to
coronary atherosclerosis, even in the absence of ischemic scarring, and that the ischemia causes the
hypertrophy. This pathologist doesn't follow this logic. If that were true, then probably you could
build muscle by holding your breath.
Remember that hypertension is also an important risk factor for atherosclerosis, stroke, and so forth.
COR PULMONALE
A quaint name for a very serious problem.
Any right-heart problem resulting from increased pulmonary vascular
resistance (usually poor ventilation, less often fibrosis or primary
vascular problems).
The cause of the poor ventilation may be anything from emphysema to a kyphoscoliosis.
"Cor pulmonale" would also include right-sided heart extra burden from narrowing of the pulmonary
vascular tree (Wegener's, pulmonary plexiform angiopathies, etc.) Whether "cor pulmonale"
includes failure due to pulmonary emboli is a question for semantics experts.
You already know how pulmonary ventilation causes increased
pulmonary vascular resistance
("causes pulmonary hypertension"). The right ventricle undergoes hypertrophy ("Feel that sternal
heave!"), dilates, loses its familiar crescent shape and becomes more rounded, and eventually fails.
The polycythemia that accompanies hypoxia make the blood more viscous and prone to clot. This
doesn't make the heart's job easier.
Increased pulmonary vascular resistance is a great impediment to a person's well-being. Often the
right ventricle's problems set the real limit on quality of life in lung disease.
NOTE: The strained right ventricle is extremely vulnerable to rhythm disturbances. Patients with
emphysema and cor pulmonale typically die very suddenly and unexpectedly as a result of an
electrical storm in their strained ventricle. This mechanism probably underlies many (if not most)
deaths from pulmonary emboli.
CONGENITAL HEART DISEASE: INTRODUCTION
Around 6-8 babies out of every 1000 has some kind of significant cardiac malformation.
Worth remembering:
Most of the time, nobody really knows why one heart forms normally and the next one doesn't. If
one sibling has a defect, the next sibling has a 5% chance of having some (not necessarily the same)
defect.
Around 5% of cardiac defects are attributed to the chromosomal abnormalities, and the others now
account for <1% of new cases.
Classic "SIDS" cases (i.e., the child was reported to be asleep)
seldom reveal a cardiac malformation, but in the much less common
situation in which a child falls dead while awake is often
caused by a cardiac anomaly (and remember long QT): J. Ped. 141: 336, 2002.
A "shunt", of course, is abnormal flow of blood from one part of the circulation to another, which
aren't supposed to communicate directly. Intracardiac shunts are usually the result of birth defects.
Remember that intra-cardiac shunts which produce turbulence are also prone to get infected
(bacterial endocarditis; why?) As with other lesions with abnormal flow, look for jet lesions where
turbulence leads to endocardial thickening, and sometimes fibrin deposition and even infection.
"Cyanosis", you remember, refers to a concentration of >5 gm/dL of unoxygenated hemoglobin in
the arterial blood. Kids who are chronically hypoxic as a result of right-to-left shunts may become
polycythemic. If the hematocrit gets about 65% or so, the blood becomes over-viscous and hard to
pump. Polycythemic blood also clots more readily.
People with right-to-left shunts develop clubbing of the digits.
You also know that
these kids are prone to paradoxical embolization (i.e., a systemic venous embolus goes directly to
the systemic arterial circulation), and particularly brain abscesses due to septic emboli.
Tetralogy of Fallot
Sketch it, and it makes sense. In the usual model, these babies have:
1. Overriding aorta (i.e., it straddles the ventricular septum, which is possible only because it has a
hole in its top)
2. Pulmonic valve stenosis (you could imagine the aorta crunching it closed; actually, there is both
stenosis of the pulmonic valve and some hypertrophy of the muscle surrounding the track out of the
right ventricle toward the pulmonary artery)
3. Ventricular septal defect (of course)
4. Right ventricular hypertrophy (obviously, since it must work so hard to perfuse the lungs through
the defective pulmonic valve).
As you'd expect, these babies have a right-to-left shunt, and are born cyanotic. The lungs are
hypoperfused (of course), and therefore won't suffer the ill-effects of hyperperfusion seen in left-to-right shunting.
The narrowed right-ventricular outflow track is the usual site of endocarditis.
Most of these people benefit from surgery.
{49021} tetralogy of Fallot
* Taussig-Bing: "Kind of the opposite of Tetralogy of Fallot", with the aorta arising from the right
ventricle and the pulmonary artery overriding the ventricle, which bears a septal defect.
Transposition of the Great Vessels: A family of disorders in which the aorta arises from the anatomic
right ventricle, the pulmonary artery from the anatomic left ventricle. Usually the aorta is anterior to
the pulmonary artery at their origin.
In uncorrected transposition, the blood flow is:
right atrium --> right ventricle --> aorta
left atrium --> left ventricle --> pulmonary artery
If the child is to survive for any length of time after birth, an atrial (usually; septum primum) or
ventricular septal defect must be present (why?).
Surgeons may be able to switch the aorta and pulmonary artery for a cure.
In corrected transposition, the atria are also switched by the malformation,
so that the blood flow is:
right atrium --> left ventricle --> pulmonary artery
left atrium --> right ventricle --> aorta
This wouldn't be a problem, except that with this setup, the tricuspid and mitral valves can't really be
normal.
{08644} transposition of the great vessels
*There are many other variants. In double-outlet right ventricle, both pulmonary artery and aorta
arise from the right ventricle.
Truncus arteriosus
The pulmonary artery and aorta are a single vessel, overriding a ventricular septal defect. Their
valve typically has four cusps, etc.
Eventually the lungs are damaged by the high-pressure flow into their arteries.
{08656} truncus arteriosus
Tricuspid atresia
"Atresia", of course, means failure of a normal lumen to develop.
Obviously, survival is only possible if there is an atrial septal defect and some other route to get the
blood to the lungs. (Think! How could this happen?)
The prognosis is still guarded. Closure of the ductus during the first few
days of life can be a catastrophe (why?)
* "Ebstein's anomaly" is a curious lesion with a low-slung leaflet and
various functional problems.
Hypoplastic left heart
Underdevelopment of the aortic and mitral valves, left ventricle, and proximal aorta.
Untreated, this is lethal shortly after birth, though compatible with normal life as an unborn child.
*One of the "great ethical debates" of the early 1990's led to the prohibition
of the use of anencephalic babies' ("Baby Theresa") hearts to treat hypoplastic left heart syndrome.
These used to save lots of lives.
Because hearts are now seldom available, about half of these kids now die
(NEJM 330: 501, 1994).
{11483} hypoplastic left heart
CONGENITAL HEART DISEASE WITH LEFT-TO-RIGHT SHUNTS ("late cyanosis")
A family of diseases in which much of the left cardiac output returns to the pulmonary system. This
eventually causes serious damage to the pulmonary vasculature.
This is bad. The left-to-right shunt will make the heart work harder, and the increased blood flow to
the lungs will eventually damage the pulmonary arteries. When pulmonary artery pressure exceeds
aortic pressure, the shunt will reverse ("Eisenmenger's syndrome"), cyanosis will occur, and death
will follow shortly.
Eisenmenger's is a major disaster. Long-term prostacyclin
(PGI2) for these people: Circulation 99: 1858, 1999.
Ventricular septal defect ("VSD", "Roger's disease" if <5 mm, etc.): A hole in the ventricular
septum. This can be serious.
90% of these are in the membranous septum. Unfortunately, this is near the bundle of His; these
patients may develop heart block.
The remaining 10% are in the muscular septum.
The best thing about VSD is that around 50% close spontaneously, either when the muscle
hypertrophies or the tricuspid leaflet adheres to the defect.
{03281} ventricular septal defect
The most extreme example is "cor triloculare biatrium", with no septum at all. (Why the name?)
*Surprisingly, these patients can do remarkably well without surgery (Am. J. Card. 77: 542, 1996).
Atrial septal defect: A hole in the atrial septum.
Among these:
90% are ostium secundum defects, problems with the foramen ovale ("patent foramen ovale", etc.).
Often this is just a probe-patent slit where the flap joins the rest of the muscle. If a flap covers such a
defect, it may re-open in the setting of pulmonary hypertension (i.e., pulmonary embolization) and
explain paradoxical embolization.
* Early surgical closure to prevent rhythm problems after
the operation: NEJM 348: 839, 1999.
5% are sinus venosus defects, near the superior vena cava. These people are likely to have one or
more of the right pulmonary veins that return to the right side of the heart, rather than the left side.
5% are ostium primum defects, low on the septum at the cardiac crux. Often these are Down's
children, and there's generally some mitral insufficiency, too.
*"Coronary sinus type" is rare. Look for a persistent left superior vena cava.
* Lutembacher's syndrome merely refers to mitral stenosis plus an atrial septal defect. What do you
think happens?
Functionally significant atrial septal defects need surgery to prevent the same
pulmonary problems
seen in VSD's.
{08498} atrial septal defect
Patent ductus arteriosus
The ductus ordinarily closes in the first few days of life. Prostaglandin E apparently keeps it open,
and closure can be induced in many cases using prostaglandin antagonists.
If the ductus fails to close, the child's "machinery murmur" needs to be turned off by surgical
ligation. If not, the same problems with left-to-right shunting seen with VSD and ASD will occur.
Note that closure can exacerbate certain cardiac birth defects. In pulmonic stenosis, transposition of
the great vessels, and hypoplastic right heart syndrome, it's best for the ductus to remain open to
supply blood to the lungs. More about this on rotations.
{08566} patent ductus arteriosus
Variations
There are a variety of "endocardial cushion defects", which suggest Down's. The most extreme
is "persistent common AV canal", in which the mitral and tricuspid valves are a single
valve, and there is an ostium primum atrial septal defect and a high VSD.
{08603} AV canal
OBSTRUCTIVE, NON-CYANOTIC CONGENITAL ANOMALIES ("acyanotic")
Coarctation of the aorta
A fibrous ring around a portion of the aorta, or perhaps another large artery. This is common in
Turner's, or may be idiopathic.
If the occlusion is pre-ductal (the rare "infantile type", i.e., proximal to the ductus arteriosus),
patents may even have heart failure while in the womb. In this case, the upper half of the body is
supplied by the aorta, and the lower half is supplied by the pulmonary artery via a patent ductus
arteriosus.
In the post-ductal (common, "adult") type, the stenosis is distal to the ductus arteriosus. Since the
lower half of the body is likely to be under-perfused (claudication, etc.), renal hypertension is usual,
and collateral arterial circulation will develop (via which artery? HINT: It notches the ribs!) If the
femoral pulses on a hypertensive patient seem late and weak, it's probably coarctation of the aorta.
You want to get "co-arc" fixed, not the least reason being that the site is prone to become infected.
Pulmonary stenosis/atresia with intact interventricular septum
Not rare. Obviously, the blood must flow to the left side of the heart (usually through an atrial
septal defect), and then to the lungs via a patent ductus.
This is a very serious lesion, and prognosis depends largely upon
how much of the right side of the heart has formed properly.
Aortic valve stenosis and atresia
In congenital valvular aortic stenosis, the valve is a fibrous diaphragm.
In congenital sub-valvular aortic stenosis, there is a thick, fibrous ring below the valve itself.
In congenital supra-valvular aortic stenosis, there's a similar ring just past the valve. * This one's
genetic. One mutation is now known to be
the elastin gene: Hum. Genet. 106:
577, 2000, Am. J. Card. 83: 1141, 1999, others.
{06779} bicuspid aortic valve
*These was recently a flap over chlamydia as contributing to the calcification of normal aortic valves,
just as they've getting blamed for atherosclerosis. I predicted
in 1996 that this will prove to be non-related, and that the bugs
were just hangers-on. This now seems to be corrct
(Am. J.
Coll. Card. 29: 1054, 1997;
Circulation 99: 2733, 1999; Mayo's Atherosclerosis 174:
337, 2004) though some groups still find a relationship
(?? immune complexes Eur. Heart J. 24: 198, 2003).
MALPOSITIONS OF THE HEART
An acardius is a birth defect in which there is no heart. If the pregnancy goes to term, it is always
because the child shares circulation with a twin.
{021109} acardius
Dextrocardia means the heart's on the right side. In Kartagener's syndrome, the cilia are immotile
due to lack of dynein arms, so the organ often end up backwards. In situs inversus totalis, with
everything backwards, the heart is usually well-formed. If the heart is the only organ that is mal-positioned, it often bears
other defects.
ENDOCARDIAL AND VALVULAR DISEASE
Valve woes: NEJM 337: 32, 1997.
Know your common etiologies!
Mitral stenosis
Old rheumatic fever
(All other causes are very uncommon)
NOTE: Isolated mitral stenosis is pretty well tolerated most of the time.
Mitral regurgitation
Old rheumatic fever
Bacterial endocarditis
Barlow's syndrome
Other birth defects at the crux
Ruptured papillary muscle (MI)
Ruptured chorda (bacterial endocarditis, Barlow's)
Dilated annulus (left CHF)
Calcified mitral annulus (maybe)
Degenerative changes of old age (the most common today: NEJM 335: 1417, 1996)
Aortic valve stenosis
Old rheumatic fever
Congenital bicuspid valve that calcified
Normal valve that calcified
Birth defects (valvular, sub-valvular)
* Alkaptonuria
NOTE: Some of the biggest hearts in clinical medicine result from aortic valve stenosis. Obviously, the
pulse pressure is narrowed, and systole is prolonged. This can have very bad consequences for
myocardial perfusion.
NOTE: As I trust you've figured out, "aortic stenosis" (by custom) refers to stenosis of the valve, not
the aorta. If a portion of the aorta is stenotic, it's called "coarctation". If the entire aorta is stenotic,
you didn't get born.
Aortic regurgitation (review Circulation 99: 1851, 1999 urges
aggressive surgical management)
Old rheumatic fever
Bacterial endocarditis
Syphilis
Dissecting hematoma / steering wheel injury
Marfan's
Fenfluramine (diet pill fiasco): about 9% of those treated got
aortic regurgitation on echo, but cardiac symptoms are not much greater
than in controls: JAMA 283: 1703, 2000.
The ring dilates:
Rheumatoid arthritis
Ankylosing spondylitis / HLA-B27 family
Syphilis
"Annuloaortic ectasia" (usually older hypertensives)
NOTE: You'll learn about increased pulse pressure, "Corrigan's jumping pulse", pistol-shot sign,
etc., etc. on rotations.
Tricuspid stenosis
Old rheumatic fever
Carcinoid heart disease
Tricuspid regurgitation
Old rheumatic fever
Carcinoid heart disease
Bacterial endocarditis (ask about IV drug use!)
Loeffler's
Dilated annulus (right CHF)
Carcinoid heart disease
NOTE: Look for those jumping neck veins!
Pulmonic stenosis
Tetralogy of Fallot
Congenital ("funnel")
Carcinoid heart disease
Pulmonic insufficiency
Dilated annulus (right CHF). Rare.
Carcinoid heart disease
Know your vegetations!
Acute rheumatic fever
Small, warty, sterile, on the lines of closure
Seldom embolize
Bacterial endocarditis
Often large, loaded with bacteria
Find them on any deformed intracardiac surface
Very prone to embolize
Non-bacterial thrombotic ("marantic") endocarditis
Small, sterile, on the lines of closure
May embolize
Libman-Sacks endocarditis of lupus and antiphosopholipid-antibody syndrome
Any size, sterile, on either surface of the leaflet
May embolize
NOTES: (1) You'll learn your murmurs in clinical medicine. "Is this a worrisome systolic
murmur?" JAMA 277: 564, 1997. (2) As with any intracardiac lesion involving turbulence,
deformed valves are prone to develop bacterial endocarditis. (3) Future pathologists: Again, look
for jet lesions on the endocardium if you suspect a valve of having produced abnormal flow.
Calcific aortic valve stenosis
While bicuspid valves are notorious for calcifying later in life, sometimes a normal, tricuspid valve
accumulates calcium-rich excrescences in its cusps. Maybe 1 older man in 100 gets at least some
of this; women are at lower risk.
*The calcification is an active process; the local cells pump out osteopontin, a bone-laying-down
protein (Circulation 92: 2163, 1995).
These can interfere with the valve opening, and can block the coronary ostia. The latter is very
serious.
{03560} calcified tricuspid aortic valve
Mitral valve prolapse ("Barlow's", "floppy mitral valve", "ballooning mitral valve", etc.)
In this semi-disease which affects around 2-3% of humankind, the mitral valve has an elongated
posterior leaflet with some extra ground substance ("myxoid degeneration"). The leaflet
looks
like a parachute canopy, and billows up into the atrium.
The other valves may show the same thing. The histopathology exhibits weird derangements of all
connective-tissue components (Am. Heart J. 129: 1149, 1995).
The typical patient has a slim build (the more "Marfanoid" you are, the most likely you are to have
"Barlow's"), a mid-systolic click (the leaflet snapping tight) and ever-so-slight mitral regurgitation.
The timing will vary with how full the ventricle gets.
It's good for these folks to take endocarditis prophylaxis prior to visiting the dentist.
If there's a stroke risk at all, it's small (Mayo Clin. Proc. 69: 632, 1994;
NEJM 341: 48, 1999).
Occasionally, they get re-entry type atrial rhythm disturbances (notably "supraventricular
tachycardia", in which one's heart "goes off like an alarm clock" at around 190 beats per minute,
making adequate filling impossible. This is moderately annoying and happens to your lecturer, who
has Barlow's, if he neglects exercise for a while and thereby drops his vagal tone). There are rumors
of people suddenly falling over dead with no abnormality except Barlow's. Is it causal? Who
knows? It's very, very infrequent.
Occasionally a chorda will rupture, producing serious mitral regurgitation.
The greatest Barlow morbidity is probably that lots of them become "cardiac neurotics". This is
really unfortunate. Natural history of Barlow's: NEJM 341: 48, 1999.
{06470} floppy mitral valve
Rheumatic fever ("romantic fever", "scarletina / scarlatina",
etc., etc.) is a systemic, autoimmune disease
triggered by a beta-hemolytic streptococcal throat infection. (The major reason for going after "strep
throat" with penicillin is to prevent rheumatic fever; around 3-5% of untreated strep throat turns into
rheumatic fever.) The disease declares itself about two weeks after the "strep throat" gets better.
Most patients are kids 5-15 years old, mostly poorer kids, (* HLA-B5 is a risk factor too; most
recently, a susceptibility gene close to HLA has been predicted Circulation 89: 138, 1994) but
nobody is immune. The disease is endemic in the poor nations (An. Int. Med. 120: 177, 1994), and
currently in resurgence in the U.S.
The classic teaching about the pathogenesis of the disease involves molecular mimicry. The M-protein of the streptococcus,
and at least one other antigen now being characterized, are attacked by
antibodies which then cross-react with the heart (anti-myosin antibody: J. Inf. Dis. 168: 915, 1993,
probably others), brain, skin, and joints.
*This can't be the whole story, of course, since some people have these antibodies without having
rheumatic fever. Perhaps the streptococcal hyaluronidase damages the endothelium, or a hapten is
involved, or....
The cardiac pathology is a pancarditis (i.e., any layer can be involved, even the pericardium). The
characteristic lesion in the myocardium (especially near the vessels)
is the Aschoff body (Aschoff nodule), a mass of fibrinoid surrounded by distinctive
Anitschkow cells ("caterpillar cells"), bloated, pale-purple-staining macrophages with a curious
caterpillar-shaped mass of heterochromatin running the length of the nucleus (in cross-section, this is
an "owl eye" instead). You may see multinucleated forms.
There's often a type III immune-injury type systemic vasculitis.
Curiously, textbooks differ about whether the Anitschkow cells are macrophages or altered
myocardial cells. Their appearance is very different from macrophages anyplace else, and this
observer thinks he sees transitional forms to nearby myocytes. However, they
stain uncharacteristically for mature myocytes, but look like they bear
immature mesenchymal nuclei (Pathology 31: 98, 1999).
Stay tuned.
* Despite the textbooks, Aschoff nodules simply do not look like
other granulomas, and I am not aware of any reason to think they should be
considered granulomas.
Here are the symptoms and signs of rheumatic fever:
Evidence of carditis (i.e., pericardial pain, enlarged heart, murmurs, failure)
Polyarthritis
Sydenham's chorea (St. Vitus's dance), from involvement of the basal ganglia. This is a big deal
right now. Mediated by antibodies that cross-react with neurons (type II immune injury, non-destructive).
Erythema marginatum, a snake-like red skin eruption
Subcutaneous nodules, little masses of fibrinoid with granulomas around them, over the bony bumps
on arms and legs.
Fever
Lab evidence of recent strep infection (i.e., a high anti-streptolysin O and/or anti-hyaluronidase
and/or anti-DNAse titer, a culture result, or whatever)
Here are the Jones criteria for making the diagnosis. You need one major and two minor, or two
majors.
Major: Polyarthritis; carditis; chorea; subcutaneous nodules; erythema marginatum.
Minor: Arthralgia; fever; preceding group A strep infection; preceding bout of rheumatic fever;
elevated sed rate; prolonged PR interval on EKG.
The inflamed heart may fail. More often, the endocarditis manifests as verrucae, tiny sterile masses
of fibrin and platelets along the lines of closure of the valve leaflets.
Unfortunately, the verrucae are prone to organize, causing deformity of the heart. If the cusps stick
together, stenosis results. If scar contraction shortens and bends the valve leaflets, insufficiency /
regurgitation results.
The aortic and mitral valves are usually affected; the tricuspid sometimes, the pulmonic rarely.
Rheumatic fever usually ends in recovery after a few weeks, but recurrences are common and likely
to be more severe.
Old rheumatic fever is one of the most common causes of cardiac valve disease.
If you suspect the cause of valve disease is rheumatic, look for fusion of the cusps, where the
verrucae have organized.
One hallmark of old rheumatic mitral valve disease is thickening of the chordae tendineae. Also,
check the posterior aspect of the left atrium for the white, geographic fibrous patches called
MacCallum's plaques; probably these are just the jet lesions from mitral insufficiency.
 Athletic heart
Athletic heart
Tom Demark's Site
 The athlete's heart is hypertrophied, often remarkably so. You can feel the apex beat far lateral to
the mid-clavicular line. The pulse is slow (50-60 beats at rest), and the QRS complex often
tremendously large (why?).
The athlete's heart is hypertrophied, often remarkably so. You can feel the apex beat far lateral to
the mid-clavicular line. The pulse is slow (50-60 beats at rest), and the QRS complex often
tremendously large (why?).
* The myosin heavy chain in hypertrophied heart is beta, rather
than alpha as in a couch potato. Slower, more efficient.
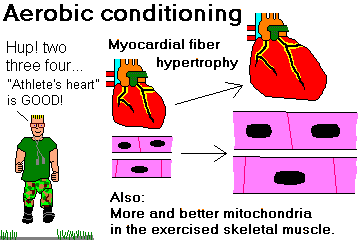
 As the heart is forced to work extra-hard, it undergoes hypertrophy (i.e., more muscle
mass) and perhaps dilatation (i.e., chamber enlargement, which helps pump the blood; remember Starling's
Law?) Eventually, however, the heart's strength cannot increase further, and the organ appears to
give up (i.e., it stops "obeying Starling's Law"). Now the chamber does not
empty fully.
As the heart is forced to work extra-hard, it undergoes hypertrophy (i.e., more muscle
mass) and perhaps dilatation (i.e., chamber enlargement, which helps pump the blood; remember Starling's
Law?) Eventually, however, the heart's strength cannot increase further, and the organ appears to
give up (i.e., it stops "obeying Starling's Law"). Now the chamber does not
empty fully.
* One team identifies urotensin II in remodelling heart (Lancet 359;
1990, 2002); maybe this is a protein involved in muscle hypertrophy; great
pictures in any case.
 Left ventricular hypertrophy
Left ventricular hypertrophy
Perhaps from hypertension
KU Collection
Concentric hypertrophy: The heart is pumping
against increased resistance, but emptying fine
(i.e., aortic valve stenosis and/or
increased systemic resistance / high blood
pressure in the absence of failure). Thick wall, chamber volume
is not excessively expanded, chamber empties.
 Coronary Artery Exhibit
Coronary Artery Exhibit
Virtual Pathology Museum
University of Connecticut
Prinzmetal's ("variant") angina is primarily attributable to vasospasm. Perhaps it's the cardiac
equivalent of migraine (if you believe migraine is caused by vasospasm).
Remember that scleroderma patients may have microvascular cardiac disease
due to the thickening of the intimal layers of the small arteries.
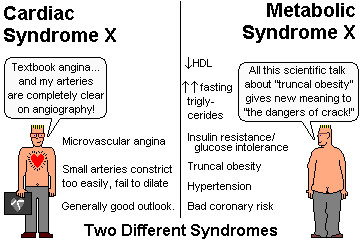
 Acute MI
Acute MI
Photo and mini-review
Brown U.
{06531} ruptured plaque with thrombus
 Coronary with atherosclerosis
Coronary with atherosclerosis
Decide yourself about obesity
WebPath photo
 Atherosclerotic coronary artery
Atherosclerotic coronary artery
Serial sections
WebPath photo
See below.
Do you believe these rather familiar autopsy findings
actually kill people? I don't know. Insurance studies show that
most people with myocardial bridges
never have trouble. Since the bridge constricts the artery
worst during systole, and since the coronaries actually fill during diastole,
you wouldn't think so... However, the anecdotal reports are interesting (Clin. Card. 20
1032, 1998).
{06587} aspergillus infection of a coronary artery Kawasaki disease
Kawasaki disease
Article and photos
AAFP
{06584} amyloid (special stain)
{17483} amyloid myocardium
{06639} very early MI (bottom only)
{06428} myocyte degeneration (hydropic change)
{06431} myocyte degeneration (hydropic change)
{06642} contraction bands
{06651} contraction bands
{06645} necrosis and polys
{06630} subendocardial MI
{06654} good necrosis and polys (NOTE: the myocyte are
homogenized rather than pyknotic; that still means "dead")
{06443} road-kill, lots of polys, fibers very dead; good contraction bands remain
{06446} nice granulation tissue
{06663} nice granulation tissue
{06666} nice granulation tissue (left)
{06449} nice scar
{06338} nice scar
{06455} nice scar (trichrome)
 Transmural infarct
Transmural infarct
Cornell
You will learn how treat myocardial infarction on rotations.
In 2004, the first reports came in from experiments in which stem
cells (from bone marrow, not embryos) were injected into the injured
myocardium. After recovery, treated patients got about 6% new muscle,
with controls getting almost none (Lancet 364: 121 & 141, 2004).
{07141} hemopericardium
{03617} ruptured septum
{53285} ruptured septum
 Ventricular aneurysm with thrombus
Ventricular aneurysm with thrombus
WebPath photo
Although there are no accepted immunologic criteria,
Dressler's is supposed
to have an
autoimmune basis, since anti-heart antibodies supposedly appear in the blood
and the response to steroids is
good.
 Extreme straining against a closed glottis while upright (i.e.,
constipation death)
Extreme straining against a closed glottis while upright (i.e.,
constipation death)
 Myocarditis (even a little patch can cause rhythm problems and
even death: "Hank Gathers's disease"); I "buy this" when you see necrotic myocytes
along with a lymphocytic infiltrate ("Dallas criteria")
Myocarditis (even a little patch can cause rhythm problems and
even death: "Hank Gathers's disease"); I "buy this" when you see necrotic myocytes
along with a lymphocytic infiltrate ("Dallas criteria")
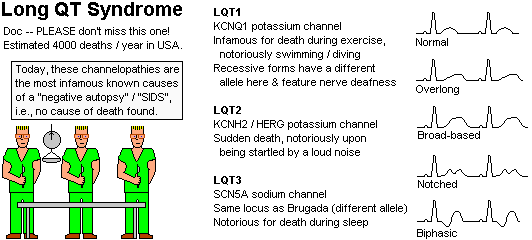
The long QT interval diseases (NEJM 339:
960, 1998; Circulation 99: 3165, 1999; stratifying risk NEJM 348:
1866, 2003; JAMA 289: 2120, 2003; Nat. Med. 10: 463, 2004).
Several different
channelopathies including some sodium channel mutations
(Circulation 101: 1698, 2000;
Circulation 102: 584 & 921, 2000) and a common
potassium channel mutation (Circulation 100: 1264, 1999).
This is now known to be a common cause of sudden death with no
anatomic findings at autopsy; this includes SIDS cases.
An episode of torsade de pointes may tip the alert
clinician, or the first sign may just be sudden death.
Updates Am. J. Med. 110: 50 & 385, 2001; Arch. Path. Lab. Med. 125:
116, 2001; Ann. Int. Med. 137: 981, 2002.
You need to be alert to these; presently the estimate is that
they cause about 4000 deaths in the US per year, i.e., around three
times as many as die of thyroid cancer.
Future pathologists: See Am. J. For. Med. Path. 22: 105, 2001
for "the molecular autopsy" (i.e., gene searches for long QT and so forth)
that follows your performing an autopsy on a young
person who has died suddenly without a previous EKG or any anatomic or
toxicologic explanation for death.
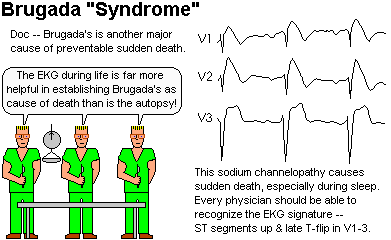
Brugada syndrome (mutated sodium channel; see below, clinical features
Circulation 99: 666, 1999; Lancet 355:
808, 2000).

While we are talking about sudden cardiac death...
When there is longstanding,
sublethal ischemia of a portion of the myocardium, the fibers
do not beat, but do not die.
If circulation is restored, the fibers reconstitute themselves
and begin beating, though it may take several months.
 CONGENITAL HEART DISEASE WITH RIGHT-TO-LEFT SHUNTS ("early cyanosis"; "blue
baby", "cyanotic congenital heart disease")
CONGENITAL HEART DISEASE WITH RIGHT-TO-LEFT SHUNTS ("early cyanosis"; "blue
baby", "cyanotic congenital heart disease")
The cause of clubbing is probably embolization of megakaryocytes
from the bone marrow. In health, many of these settle in the lungs.
Where there is a right-to-left shunt through the pulmonary circulation
(i.e., congenital heart disease,
lung tumor, TB, granulation tissue), megakaryocytes get into the
systemic circulation, and because
they are large they tend to take the straight-shot to the fingers and toes.
Evidence comes from histopathology (i.e., clubbed digits are
packed with megakaryocytes), and from the fact that people with patent ductus
arteriosus causing Eisenmenger's get clubbing of the toes but not the fingers.
See Am. J. Card. 86: 1198, 2000.
{08627} tetralogy of Fallot
{08630} tetralogy of Fallot
{08483} clubbing
{08647} transposition of the great vessels
{08650} transposition of the great vessels
{40920} transposition of the great vessels
 Transposition of the great vessels
Transposition of the great vessels
Pittsburgh Pathology Cases
 Truncus
Truncus
WebPath photo
 *"Baby Fae" was born with a hypoplastic left heart.
She was given a baboon's heart by a surgeon interested in "independent
scientific thought".
Do you remember the press conference in which the surgeon predicted she'd live
a normal life? Most people would consider the whole business unethical.
I think that reasonable people can disagree about this.
*"Baby Fae" was born with a hypoplastic left heart.
She was given a baboon's heart by a surgeon interested in "independent
scientific thought".
Do you remember the press conference in which the surgeon predicted she'd live
a normal life? Most people would consider the whole business unethical.
I think that reasonable people can disagree about this.
* Pregnancy, with increased blood volume, speeds this up.
{03287} ventricular septal defect (sorry about ruler over hole)
{03635} membranous ventricular septal defect
{03638} membranous ventricular septal defect
{08525} ventricular septal defect
{03650} complete atrioventricular septum defect
 Eisenmenger's, lung
Eisenmenger's, lung
Lung pathology series; follow the arrows
Dr. Warnock's Collection
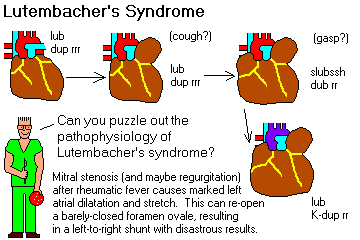
{08501} atrial septal defect
{08513} atrial septal defect
{08569} patent ductus arteriosus
{08617} AV canal
 Milder birth defects of the aortic valve are much more common. Bicuspid aortic valve, seen in
around 1% of folks, produces problems only because it tends to calcify later in life, and become
stenotic. Unicuspid aortic valve, surprisingly, also works. Tetracuspid valve is a curiosity.
Milder birth defects of the aortic valve are much more common. Bicuspid aortic valve, seen in
around 1% of folks, produces problems only because it tends to calcify later in life, and become
stenotic. Unicuspid aortic valve, surprisingly, also works. Tetracuspid valve is a curiosity.
{46205} bicuspid aortic valve
{06818} bicuspid aortic valve, this had both stenosis and insufficiency
{06785} unicommissural aortic valve
{53319} aortic valve with four cusps
 Bicuspid aortic valve
Bicuspid aortic valve
WebPath photo
{21110} acardius
The page [Lydgate] opened on was under the head[ing] of Anatomy,
and the first passage that drew his eyes was on the valves of the heart.
He was not much acquainted with valves of any sort, but he knew that
valvae were folding-doors, and through this crevice came a sudden
light startling him with his first vivid notion of finely adjusted
mechanism in the human frame... The moment of vocation
had come, and before he got down from his chair, the world was made new to him...
From that hour Lydgate felt the growth of an intellectual passion.
-- George Eliot, "Middlemarch"
 Endocardium Exhibit
Endocardium Exhibit
Virtual Pathology Museum
University of Connecticut
 Valve Exhibit
Valve Exhibit
Virtual Pathology Museum
University of Connecticut
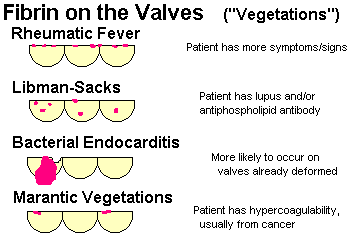
{06461} calcified tricuspid aortic valve
{40363} floppy mitral valve
{03440} floppy aortic valve, from a Barlow's case
* The autoantibody, its target, and the strep protein which evokes it
have been identified (Nat. Med. 9: 823 & 914, 2003.
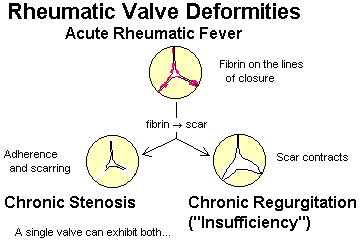
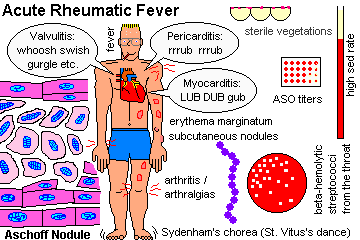
 {11489} old rheumatic fever, mitral valve
{11489} old rheumatic fever, mitral valve
{11492} acute rheumatic fever
{49008} acute rheumatic fever, vegetations
{18809} Aschoff nodule
{28587} Aschoff nodule
{28590} Aschoff nodule
{53304} Aschoff nodule
{46305} Aschoff nodule
{46481} Aschoff nodule, great caterpillar chromatin
{06467} old rheumatic fibrosis of mitral valve
{06845} old rheumatic mitral stenosis
{06847} old rheumatic mitral stenosis
{06850} old rheumatic mitral stenosis, large left atrium
{06815} old rheumatic aortic valve disease with insufficiency
{11549} old rheumatic aortic valve disease, good fusion of cusps
{11486} old rheumatic aortic valve stenosis
|
|
 Old Rheumatic Fever
Old Rheumatic FeverAustralian Pathology Museum High-tech gross photos
|
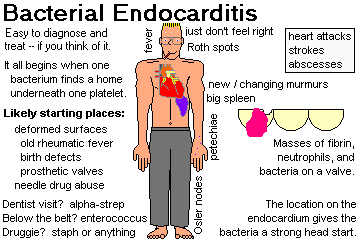
Infective (bacterial) endocarditis (Lancet 363: 139, 2004.
Blood flow in the normal heart is laminar. If the flow becomes turbulent (i.e., usually due to congenital or rheumatic deformities), endothelium is likely to be damaged.
If endothelium is damaged, tiny platelet-fibrin clots can form on the valves. This wouldn't be much of a problem, and they'd probably go away, except for that fact that every once in a while, a bacterium can get enwrapped in the clot.
Yes, there's often a bacterium or two in your bloodstream. If you brush your teeth or pass stool, you're going to get a few bacteria into your blood. If you go to the dentist or get catheterized, proctoscoped or cystoscoped, or have colon cancer, you'll get lots more. All of these place you at risk for endocarditis. And if you do IV drugs without using sterile technique, you're begging for trouble.
Once the bacterium has found a place to live under the fibrin cocoon, it is relatively safe from the body's defenses, since the valve leaflets are avascular, and the blood in the chamber is flowing too fast to allow neutrophils to stop here. The surrounding endocardium will probably get damaged, and the vegetation can grow.
Sooner or later, the polys will notice the bacteria and start fighting them, but by this time, they are generally massive. They are going to cause problems.
Naturally, the foul breakdown products of these bacteria are going to make a person systemically sick. Fever, arthritis, the acute phase reaction, anemia of chronic disease, diffuse proliferative glomerulonephritis, evidence of B-cell hyper-activation (rheumatoid factor, cryoglobulins, false-positive syphilis test), clubbing, etc., etc., are prone to supervene.
The vegetations are notoriously crumbly ("friable"; why might this be? Hint: polys), and prone to embolize. Embolic phenomena include splinter hemorrhages of the nails (if they are in the proximal nailbed I'd worry), Osler's nodes (sore spots in the finger pulp), and Roth spots (vasculitis where arterial branch-points have caught septic emboli, seen in the eyegrounds). More serious are infarcts (strokes, heart attack, and so forth) and the development of mycotic aneurysms and/or abscesses throughout the body.
Endocarditis is rightly dreaded. Even nowadays, about 1 patient in 6 will die despite everything you can do (JAMA 288: 75, 2002).
|
|
|
|
 Infective Endocarditis
Infective EndocarditisAustralian Pathology Museum High-tech gross photos
|
The vegetations (actually, probably the polys) are prone to eat through valve cusps and chordae, causing further damage and even perforation.
Acute bacterial endocarditis may affect a normal valve, is often caused by virulent staphylococci (less often, beta-hemolytic streptococci, gonococci, * proteus, * pseudomonas), and is highly lethal (death in less than 6 weeks). These people may be IV drug abusers or have a severe infection elsewhere (especially if the infection is on the right side; the higher pressures, greater turbulence, and higher oxygen concentrations make the left side more popular with most bacteria). Or they have occult colon cancer. Or they may just be unlucky.
Subacute bacterial endocarditis generally affects cardiac surfaces where there's been turbulent blood
flow. The usual culprit is viridans α-hemolytic streptococci; enterococci or
gram-negatives are
common if the person's had a below-the-belt medical procedure. Actually, lots of bacteria, as well as
candida and aspergillus (Arch. Path. Lab. Med. 129: 511, 2005), can produce subacute bacterial endocarditis.
The distinction between acute and subacute is kind of artificial, of course.
Clinicians spend a great deal of time looking for endocarditis, which is good. It's notoriously
subtle in producing symptoms, it is treatable, and it is fatal if untreated. The mainstay of making the
diagnosis is the positive blood culture, coupled with findings on daily meticulous physical exam
(changing murmurs, petechiae, etc.)
If some Hippocrates has given your patient antibiotics "for that fever", don't expect to grow the
bacteria even if the vegetations are massive.
*Q-fever endocarditis: Am. J. Med. 100: 629, 1997. Diagnosis by serology.
{06563} acute bacterial endocarditis
{08458} acute bacterial endocarditis
{38563} bacterial endocarditis
{03293} bacterial endocarditis, aortic valve cusp perforation
{03413} bacterial endocarditis, gross
{06986} bacterial endocarditis
{06989} bacterial endocarditis
Non-bacterial thrombotic endocarditis ("marantic endocarditis")
Some people who have cancer (notably of the pancreas), or are otherwise super-sick with
hypercoagulable blood, or who aren't, may develop little fibrin-platelet thrombi, without bacteria, on
the lines of valve closure.
Sometimes these contain a few polys, but usually they don't. Sometimes they embolize, but usually they don't.
{06326} Non-bacterial thrombotic endocarditis
Lupus endocarditis ("Libman-Sacks")
Lupus and/or the antiphospholipid syndrome (Circulation 93: 1579, 1996) can result in small
masses of fibrin on various valve surfaces and anywhere else on the endocardium.
It seems reasonable to think that these are foci of type III immune injury. Be this as it may, you
might spot a hematoxylin body here.
Valves are seldom damaged or caused to malfunction, but occasionally the vegetations break off and
embolize.
{06962} Libman-Sacks endocarditis
Calcification of the mitral annulus
A common, minor problem seen in many older patients. While various rhythm disturbances and
mitral insufficiency are attributed to this lesion, most often it seems harmless.
Carcinoid heart disease
Carcinoids which release their products into the great veins (i.e., those in the ovary or metastatic in
the liver) often produce endocardial fibrosis, generally limited to the right side of the heart (i.e.,
whatever does it is "detoxified" in the lung). The tricuspid and pulmonic valves may malfunction, with
tricuspid regurgitation being the usual finding.
Though much of "carcinoid syndrome" is caused by serotonin and bradykinin, it's still
not clear which molecules are responsible. The Fenphen fiasco
has focused attention back on serotonin, and the higher the 5-HIAA (serotonin metabolite)
secretion in the urine, the worse the heart disease (NEJM 348: 1005, 2003).
{08044} carcinoid heart disease (trust me; look for the
white fibrous stuff)
Endocardial fibroelastosis
Some infants in the U.S. develop endocardial fibroelastosis, a tough thickening of the endocardium
that ultimately interferes with the heartbeat. The cause remains obscure; there are rumors of a link
to mumps.
In central Africa, there's a relatively common, similar lesion centered on the apex. In the past, this
has been blamed on eating little green bananas ("rich in serotonin, mimicking the carcinoid
syndrome"); however I understand these are also a staple in the Caribbean and that the disease isn't a
problem there. I have been unable to find anything recent about its likely causes.
{10739} endocardial fibroelastosis
* Whipple's endocarditis is a fibrosing process
caused by Tropheryma whipplei which is familiar from the gut.
Bacteria fill macrophages which in turn fill and deform the endocardial tissue
with resulting scarring.
See J. Inf. Dis. 190: 935, 2004.
Q-fever endocarditis, caused by the rickettsia, is also well-known
(J. Inf. Dis. 187: 1097, 2003.
Complications of artificial valves: common and serious.
Nobody makes valves as good as the Good
Lord's, but sometimes the originals do need to be
replaced. Ask a cardiac surgeon about these problems.
{53323} Bjork-Shiley "toilet seat"/"disk" valve
{03626} infection on prosthetic valves
MYOCARDITIS
Inflammation of the heart. The heart muscle itself is incredibly resistant to bacterial infections
unless they extend from endocarditis. Most myocarditis is probably viral and/or autoimmune.
The only systemic bacterial infection that is likely to produce a neutrophilic
myocarditis is meningococcemia, and this seems to be the exception even with this dread
micro-organism. The well-known negative inotropic effect in meningococcemia now seems
to be mediated by interleukin 6 (Lancet 363: 203, 2004).
Most worthy of note are coxsackie A and coxsackie B virus infections (look for single-fiber
necrosis; remember the selenium deficiency in China made people much
more vulnerable to this), diphtheria (remember the toxin?), trichinosis (look for eosinophils, even if you see no
worms), anthracyclines (adriamycin-doxorubicin; recently appreciated Am. J. Clin. Path. 100: 158,
1993; Ann. Int. Med. 125: 40, 1996) and Chagas' disease (look for the T. cruzi bugs in the heart
cells, and/or an "autoimmune" cardiomyopathy which has to date not yielded up its secrets: Am. J.
Card. 79: 1135, 1997).
*Also worth mentioning are the rickettsial diseases: typhus, Rocky Mountain spotted fever, Q-fever.
For research, you'll want to see necrosis of myocaridal fibers
plus a lymphocytic infiltrate to make the call of "active myocarditis" ("the Dallas criteria"). You may find abscesses in the heart muscle in people dying
of sepsis, or with severe diabetes.
{24946} coxsackie B myocarditis
In most of these forms of myocarditis, look for a lymphocytic infiltrate coupled with various kinds of
damage to myocardial fibers.
Loeffler's eosinophilic disease of the heart usually features fibrosis
of the endocardium with a lot of eosinophils.
Sarcoidosis and "idiopathic giant-cell
myocarditis" are other important inflammatory diseases of heart muscle.
Don't confuse them; in the latter, the giant cells are probably of muscle
origin. Sarcoidosis identified on heart biopsy is ominous (Am. Heart J. 150:
459, 2005). It's likely that the latter is a reaction pattern, at least sometimes
caused by autoimmunity, very deadly and with variable responses to
immunosuppression.
{07946} sarcoid heart
Also remember: As you reject your heart transplant, it will become inflamed.
As you would expect, all lead to a dilated, failing heart in severe cases.
CARDIOMYOPATHIES: Non-inflammatory, non-ischemic heart muscle disease. (Call the heart
transplant team!) Review: Br. Med. J. 315: 1529, 1997.
Arrhythmogenic Right Ventricular Cardiomyopathy (J. For. Sci. 42:
32, 1997; Circulation 94: 983, 1996; progressive nature of the lesion
with apoptosis Am. J. Path.
152: 479, 1998; sudden death in vigorous good health
AJFMP 18: 345, 1997).
The pathologist sees a curious mix of fatty ingrowth and fibrosis
in the wall of the right ventricle (sometimes the left also or instead). We're working out the pathology
and mechanism. The cell pathology is bizarre (Am. J. Med. Sci. 320:
310, 2000).
First series of 200 deaths: Circulation 108: 3000, 2003.
Dilated ("flabby heart")
{03629} dilated cardiomyopathy vs. normal
There are a host of lesions which cause the heart muscle to lose its strength. There's a list in "Big
Robbins"; most cases never get a diagnosis (NEJM 331: 1564, 1994).
Alcoholic's heart
Alcohol is rough on most organs, and the liver and brain damage take precedence most of the time to
the cardiac toxicity.
Alcohol may or may not have a direct toxic effect on the heart, by itself or through a metabolite.
Lots of alcoholics are deficient in vitamin B1, and this can produce high-output failure or even
weaken the muscle.
In 1966, some genius added cobalt to Quebec beer to make a nicer head of foam. Cobalt poisons
pyruvate metabolism and produced a beriberi-like cardiomyopathy. (*The classic
pathology: Ann. N.Y.Acad. Sci. 156: 577, 1969).
Pregnancy
Around the time of parturition, some women get a dilated cardiomyopathy.
This "peripartum cardiomyopathy" is very serious: JAMA 283:
1183, 2000 (NIH conference).
Sadly, this is mostly a disease of poor women, especially those who have had several babies close to
one another. Perhaps it is the result of a deficiency in some trace nutrient. It remains mysterious:
Am. Heart J. 130: 860, 1995; and it's likely to recur: NEJM 344: 1567, 2001.
Acromegaly (apoptosis of myocytes for some reason): Circulation 99:
426, 1999.
Lots of people with "end-stage idiopathic dilated cardiomyopathy with lymphocytes" have some lab
evidence of autoimmunity against the heart (specifics vary, and will bewilder you), and "a possible
history of viral myocarditis". Actually, the link between this entity and viral infection is pretty
weak. Many of these people have antibodies against coxsackie viruses,
grow enteroviruses or adenoviruses from heart muscle
(Circulation 99: 1348, 1999) etc., etc., and nobody has
been able to come up with anything more convincing.
* To nobody's surprise, the lymphocytes turn out to be T-cells, and unlike those in a normal person's
heart, most of these seem to be active (Circulation 92: 2876, 1996).
* Update on autoimmunity and viruses, and how they may work together,
in causing "idiopathic cardiomyopathy": Nat. Med. 9: 1448, 2003.
Genetic syndromes: Syndromes
worth remembering include
Friedreich's ataxia and Duchenne's-Becker's muscular dystrophy.
Even female carriers of the latter often get a dilated cardiomyopathy
(JAMA 275: 1335, 1996). There are some other syndromes which
give only a dilated cardiomyopathy (* one is due to mutant actin:
Circulation 99: 1022, 1999; others to mutant troponin T
(pathology Circulation 104: 1380, 2001),
and
beta-myosin heavy chain NEJM 343: 1688, 2000; mutated desmin
affects skeletal and cardiac muscle NEJM 342: 770, 2000;
phospholamban Science 1410, 2003; troponin I: Lancet 363:
371, 2004 is unusual for being recessive).
Remember sarcoidosis, Chagas, radiation, giant-cell myocarditis,
rheumatic fever, ipecac (munchausen's by proxy: Pediatrics 97: 902,
1995).
Hypertrophic ("idiopathic septal hypertrophy", "asymmetric septal hypertrophy"; "Les Aspin's
disease"; "Reggie Lewis's disease"; "muscle-bound heart";
Hank Gathers
supposedly had myocarditis rather than hypertrophic cardiomyopathy
NEJM 329: 55, 1993)
{07811} hypertrophic cardiomyopathy
This curious lesion, generally autosomal-dominant, exhibits (1) variable regions of hypertrophy (in
contrast to the "concentric hypertrophy" of left ventricular overwork
and the "eccentric hypertrophy" of left ventricular overfilling); (2) disarray of cardiac muscle
cells by light and electron microscopy; (3) fibrosis around muscle cells; (4)
some fibrous narrowings of the small arteries.
This is serious. The outflow tract from the left ventricle toward the aortic valve is likely to be
obstructed by the large mass of muscle ("obstructive hypertrophic cardiomyopathy"; "HOCM").
Be careful! If you do aerobic exercise and develop your heart muscle, you're likely to make this
particular problem worse. (Here's the case for routine auscultation of the heart in would-be
athletes. See J. Fam. Pract. 52: 127, 2003 for the pre-sports physical,
author recommends what I've always done and taught.) The septum typically
takes its thick shape in the teenaged years.
Most cases are autosomal-dominant, and a majority are
caused by a mutated beta-myosin gene. Mouse with the
mutation: Science 271: 663, 1996. Other loci are now known, mutant troponin T is common
and especially deadly (histopathology Circulation 104: 1380, 2001), and
tropomyosin and myosin binding protein
are rare (NEJM 332: 1058, 1995; J. Am. Coll. Card. 29: 635, 1997;
NEJM 338: 1249, 1998; Lancet 355: 58, 2000).
Many patients die suddenly in youth. But
many others do not die of the disease, or die of it in
old age (CHF or rhythm problems: JAMA 281: 650, 1999).
* When in doubt about how to manage this, put the patient on a treadmill. Dropping the blood
pressure is a warning of more ominous things to come.
The risk for sudden death cannot be predicted simply by measuring
the thickness of the wall (Lancet 357: 420, 2001). Gadolinium scan to
predict risk: J. Am. Coll. Card. 41: 1561, 2003.
The implantable defibrillator for these people: NEJM 342:
422, 2000.
* The name of Reggie Lewis will always be associated
with hypertrophic cardiomyopathy. However, this was not found on ultrasonography
in life, or at autopsy. The extensive scarring described at autopsy
sounds much more like "cocaine heart".
During the litigation, which finally ended in 2005,
there was a great deal of lawyering by attorneys for his family
about Lewis's failing and/or refusing
to take drug tests for cocaine.
Of course, we'll never know for certain.
Restrictive-infiltrative-obliterative ("stiff heart"; amyloid)
Most older people get some amyloids of various compositions (known and unknown) in their atria
and aortas. All about amyloid and the heart: J. Clin. Path. 58: 125, 2005.
If amyloid involves the myocardium extensively, the muscles cannot contract. This is the usual
cause of "restrictive cardiomyopathy".
* Amyloid hearts bounce; I was shown this once. Don't try it; it's disrespectful.
* There are a few poorly-understood restrictive cardiomyopathies of childhood,
with collagen rather than amyloid.
{07937} amyloid heart
Secondary cardiomyopathies: Hemochromatosis (dysrhythmogenic!), pheochromocytoma
("catecholamine heart", with single-fiber necrosis as in cocaine heart), chemotherapy (doxo- and
daunorubicin; "Adriamycin"; flabby dilated heart), Pompe's glycogenosis, Duchenne's & Friedreich's
(as above), end-stage HIV infection (Br. Med. J. 309: 1605, 1994).
{07039} von Gierke's disease
* CADAVERIC SPASM
This is instantaneous, permanent stoppage of the heart, due to extreme pain or fright.
There are reports from the battlefield of soldiers found dead, or seen to die, with no injury, but with
their muscles frozen in position. The cause was terror; the mechanism must be influx of calcium
into skeletal and cardiac muscle, as in rigor mortis.
If cadaveric spasm really occurs, it must be rare. It makes for good stories, though. It's also the
origin of the comforting untruth that, if you fall or jump off a building or something and see that you will
surely die, your heart will stop before you hit the ground.
PERICARDIAL DISEASE (Lancet 363: 717, 2004)
Pericardial effusions
Many different situations result in accumulation of fluid (i.e., more than the usually few mL) in the
pericardial sac. Viral pericarditis is often accompanied by a serous effusion; TB's effusion may also
be serous.
However, to produce tamponade, it takes around 150 mL of fluid, and it must build up pressure
rapidly (i.e., hemopericardium after ventricular rupture, penetrating trauma, or aortic dissection; pus
in a suppurative pericarditis). If the fluid accumulates slowly, the pericardium can expand. I've
seen a one-quart effusion that didn't seem to cause a problem.
On rotations, you'll learn how to do pericardiocentesis, and to place a pericardial window to keep the
fluid "off the heart".
Pericarditis
Inflammation of the pericardium hurts. Patients typically get relief by leaning forward. You, the
physician, will strain to hear the notoriously ephemeral "friction rubs" of this illness. In sporadic
pericarditis, the etiologic agent is probably some virus or immune phenomenon. It usually is self-limited;
today people treat it with NSAIDs.
"Fibrinous pericarditis" (the familiar "bread and butter", or more realistically, "ketchup on bread"
lesion) results from myocardial infarction (over a transmural infarct; Dressler's), uremia, radiation,
lupus, rheumatic fever, surgery, and trauma. It may organize (obliterating the pericardial space, which isn't
usually a big deal), or even calcify (perhaps limiting distensibility).
{18719} fibrinous pericarditis
Pus in the pericardial space usually means bacterial infection. They usually got there by way of a
nearby pneumonia or empyema, though there are other routes. Again, it may organize (a few
adhesions, or "obliterative pericarditis"; or even calcify. TB in particular is infamous for causing
troublesome scars. Old healed pericarditis may become "constrictive" and cause problems.
"Hemorrhagic pericarditis" is due almost exclusively to TB and cancer. Caseous material in the
pericardial sac is TB.
{03659} constrictive non-calcific pericarditis
* The "soldier's plaque" or "milk spot" on the visceral pericardium (i.e., the epicardial surface) is a
focus of very slight thickening of the fibrous layer under the mesothelium.
This mysterious finding is harmless. It was once attributed to packs worn during WWI, until some clever
person
noted that it's equally common in women. Today, somebody may tell you it represents "healed
pericarditis", but it doesn't really look like scar.
CARDIAC TUMORS are rare.
The principal primary heart tumor is the myxoma, a lesion of endothelial origin that arises as a ball
from the atrial septum and fills the left atrium. Grossly, it's a typically benign, soft tumor.
Microscopically, there are lots of spindle cells and ground substance, and few vessels. Myxomas
(familiarly called "wrecking balls") can plug the mitral valve (instant death), or cause emboli
(under-recognized, Chest 107: 674, 1995).
We recently figured out that atrial myxoma are really tumors, not just organized atrial "ball-valve"
thrombi of the sort seen in mitral valvular disease.
*As genuine tumors, there's an anti-oncogene deletion syndrome ("Carney complex", with lentigos
too, and others: Am. J. Card. 79: 994, 1997).
All about cardiac myxomas: Am. J. Clin. Path. 100: 671, 1993; NEJM 333: 1610, 1995.
{07975} myxoma
People with tuberous sclerosis are prone to have small cardiac hamartomas ("rhabdomyomas").
These are seldom troublesome.
I was amazed by my first papillary fibroelastoma (J. Thor. Card.
Surg. 112: 551, 1996; fatal
AJFMP 19: 162, 1998) at a 1991 autopsy. It looked like a
sea anemone on the pulmonic
valve.
They can cause embolic stoke (Clin. Card. 24:
346, 2001); look for them using transesophageal echocardiography.
Series Circulation 103: 2687, 2001.
Lipomas ("lipomatous hypertrophy" / "massive
fatty deposits") of the septum are occasionally seen
(review AJFMP 17: 43, 1996); it can be left alone.
* "Cystic tumor of the AV node" is a hamartoma
that mimics the ultimobranchial nests of the thyroid (Am. J. Clin. Path. 123: 369, 2005).
{08029} lipomatous hypertrophy ("lipoma") of atrial septum
Metastases to the heart are usually from lung, breast, melanoma, lymphoma, leukemia, and
choriocarcinoma. Any of these can be troublesome (heart block, muscle failure, effusion), but you
will usually miss the diagnosis during life.
LAST PHOTOS
{17416} tiger-stripes of fatty change in extreme anemia; remember this one?
PULSUS PARADOXUS OVERSIMPLIFIED
You need to understand this. In health, venous pressure drops slightly during inspiration because
the decreased intrathoracic pressure draws venous blood into the chest. The pressure drop is less
than 10 torr and is not obvious on palpation.
In health, arterial pressure drops slightly during inspiration because the greatly expanded pulmonary
vascular bed takes up the right cardiac output, diminishing return to and output from the left.
In restrictive pericardial disease, the diaphragm pulling down on the pericardium pulls it even
tighter. This further blocks venous return to the heart, so that venous pressure actually increases
during inspiration. It also blocks arterial outflow from the heart, greatly accentuating the normal
drop in arterial pressure during inspiration (>10 torr, should be obvious on palpation).
 Bacterial endocarditis
Bacterial endocarditis
WebPath photo
{06965} Non-bacterial thrombotic endocarditis
{53315} Non-bacterial thrombotic endocarditis
{53317} Non-bacterial thrombotic endocarditis
 Aortic valve vegetations
Aortic valve vegetations
Probably marantic thrombi
KU Collection
 Libman-Sacks Endocarditis
Libman-Sacks Endocarditis
Thailand
 Calcified mitral valve annulus
Calcified mitral valve annulus
WebPath photo
{08047} carcinoid heart disease (pulmonic valve, trust me)
{07732} prosthetic valve endocarditis
{07723} worn-out prosthetic valve
* A horrifying account of coxsackie myocarditis and deaths
in a cargo box full of illegal immigrants: Am. J. For. Med. Path. 25: 117, 2004.
* More on the Dallas Criteria: Hum Path. 18: 619, 1987.
More recently the World Heart Federation suggests fourteen or more white
cells per square millimeter of slide, plus necrosis or degenerative changes in muscle fibers.
 Viral myocarditis
Viral myocarditis
Photo and mini-review
Brown U.
{03461} myocarditis, microscopic
{15753} Chagas' disease
{26582} Chagas' disease
{53320} toxoplasmosis
* Acute eosinophilic myocarditis is a spectacular, deadly
illness in which eosinophils massively infiltrate the heart and necrosis
occurs (Heart 81: 669, 1999).
*Idiopathic giant cell myocarditis: Mayo Clin. Proc. 74: 1221, 1999.
In humans, the eye muscles are prone to be involved also.
The animal model involves immunizing with myosin.
 Sarcoid heart
Sarcoid heart
Fatal case
Loyola Med
 Idiopathic giant cell myocarditis
Idiopathic giant cell myocarditis
Pittsburgh Pathology Cases
If death is due to heart failure, expect to see widespread apoptosis of heart
muscle cells (Am. J. Card. 94: 746, 2004). They will be less prominent if
death is due to a rhythm problem.
 Cardiomyopathy photos
Cardiomyopathy photos
Cardiomyopathies
Thanks Dr. Kasper
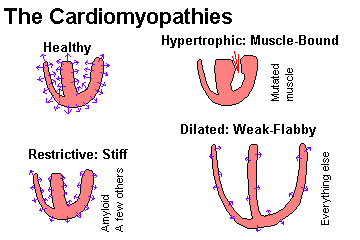
"Arrhythmogenic right ventricular dysplasia" ("fibrolipomatosis")
Recently-described cause of sudden death, especially in the young,
especially the athletic young but seldom actually while exercising. If heralded at all, it's most likely
been only by mild rhythm problems.
Two genes are known (cardiac ryanodine receptor 2 / ARVD2 and desmoplakin ARVD8: Circulation 109: 1180, 2004.
 Arrhythmogenic Right Ventricular Dysplasia
Arrhythmogenic Right Ventricular Dysplasia
Tabib's paper from circulation
Dramatic transillumination photo
{03632} dilated cardiomyopathy
{07769} dilated cardiomyopathy
{11555} dilated cardiomyopathy
 "Post-viral", allegedly autoimmune ("Barney Clark's disease").
"Post-viral", allegedly autoimmune ("Barney Clark's disease").
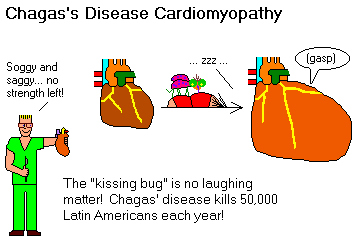
{07814} hypertrophic cardiomyopathy
{07799} hypertrophic cardiomyopathy
{07820} hypertrophic cardiomyopathy, microscopic
{11561} hypertrophic cardiomyopathy, microscopic
{07823} hypertrophic cardiomyopathy, electron micrograph

* Two genes for a very severe form, lethal early in life, are
known (Am. J. Med. Genet. 72: 101, 2003); one is cytochrome oxidase
itself and the other is one of its assembly proteins.
* Hypertrophic cardiomyopathy is the most common cause of sudden
death in competitive athletes, by a substantial margin (JAMA 276: 199, 1996).
* The pathology at death correlates with the type of death. As you'd expect,
the more fibrosis, the greater the risk of failure and late rhythm problems.
The more fiber disarray, the greater the risk of sudden death during sports in youth:
Am. J. Coll. Card. 88: 275, 2001.
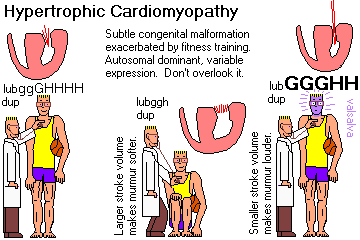
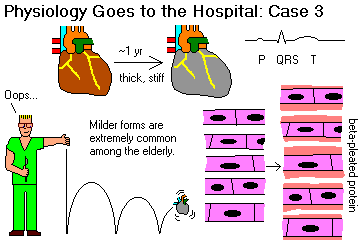
{07940} amyloid heart
{07943} amyloid heart
{07958} hemochromatosis
{07967} Fabry's
{08053} pheochromocytoma heart
{08078} radiation atherosclerosis
{08081} radiation pericarditis
 Hemochromatosis
Hemochromatosis
WebPath photo
* One new cause is getting your first smallpox immunization (vaccinia
myopericarditis, about 1:12,000 recipients): JAMA 289: 3283, 2003.
 Fibrinous pericarditis
Fibrinous pericarditis
Classic gross appearance
KU Collection
 Suppurative pericarditis
Suppurative pericarditis
WebPath photo
{07982} myxoma
{11480} thromboembolus caught in heart

FINISHING UP
* The desires of the heart are as crooked as corkscrews.
--W.H. Auden
 By now, one would have to be very naive to think
that most of his or her neighbors (patients, whatever) don't prefer death
to severe and expensive disability. This preference is showcased in a study (NEJM 330: 545, 1994)
in which a group of older patients were told the (abysmal) odds of surviving CPR reasonably intact.
The vast majority never-ever wanted it done to them. CPR doesn't work for
trauma cases (why not?), and even in ischemic heart disease,
the few cases where it does work are very likely to leave the "survivor"
badly brain-damaged.
Really, one's only hope is rapid defibrillation, which thankfully is now
being emphasized (NEJM 348: 2626, 2003).
When the geriatricians trashed "ER" for
showing an excessively-high rate of good outcomes of CPR (NEJM 334: 1578, 1994), the producers
shot back that it's the doctors' fault that patients hear about CPR from, and only from, Hollywood
(no kidding, NEJM 334: 1578, 1994). As a physician, I welcome signs that the "keep them all alive
as long as you can, you make more money" era is finally coming to an end. As a physician, I'm
sorry that most of the impetus for change hasn't come from my fellow-physicians.
By now, one would have to be very naive to think
that most of his or her neighbors (patients, whatever) don't prefer death
to severe and expensive disability. This preference is showcased in a study (NEJM 330: 545, 1994)
in which a group of older patients were told the (abysmal) odds of surviving CPR reasonably intact.
The vast majority never-ever wanted it done to them. CPR doesn't work for
trauma cases (why not?), and even in ischemic heart disease,
the few cases where it does work are very likely to leave the "survivor"
badly brain-damaged.
Really, one's only hope is rapid defibrillation, which thankfully is now
being emphasized (NEJM 348: 2626, 2003).
When the geriatricians trashed "ER" for
showing an excessively-high rate of good outcomes of CPR (NEJM 334: 1578, 1994), the producers
shot back that it's the doctors' fault that patients hear about CPR from, and only from, Hollywood
(no kidding, NEJM 334: 1578, 1994). As a physician, I welcome signs that the "keep them all alive
as long as you can, you make more money" era is finally coming to an end. As a physician, I'm
sorry that most of the impetus for change hasn't come from my fellow-physicians.
*Transgenic pigs are now being bred as heart donors: Br. Med. J. 312: 657, 1996. Since then, there has been a great deal of work, but with publications only in the super-specialty literature. Stay tuned.
You'll hear in ACLS about electromechanical dissociation, i.e., the EKG is fine and the heart isn't pumping. In other words, dead with a normal EKG. What would cause this? HINT: Pulmonary emboli, tamponade, no venous return to the heart because of bleeding or widespread vasodilatation, ruptured septum, no calcium. Understand the "why" of each? Can you think of others? Since the only one that you're going to be able to treat during CPR is "no calcium", you'll give calcium by vein. Don't expect....
* A final, personal note: The heart is a source for metaphor. My favorite quotations concerning the heart include Jeremiah 17: 9-10 and 31:33 and Koran 91:4. See also the Katha Upanishad for a classic description of the metaphysical heart. And of all the experiences in clinical medicine, the one which this pathologist misses the most is the sound of the beating heart.

|
|
| Visitors to www.pathguy.com reset Jan. 30, 2005: |
Ed says, "This world would be a sorry place if
people like me who call ourselves Christians
didn't try to act as good as
other
good people
."
Prayer Request
 Teaching Pathology
Teaching Pathology
PathMax -- Shawn E. Cowper MD's
pathology education links
Ed's Autopsy Page
Notes for Good Lecturers
Small Group Teaching
Socratic
Teaching
Preventing "F"'s
Classroom Control
"I Hate Histology!"
Ed's Physiology Challenge
Pathology Identification
Keys ("Kansas City Field Guide to Pathology")
Ed's Basic Science
Trivia Quiz -- have a chuckle!
Rudolf
Virchow on Pathology Education -- humor
Curriculum Position Paper -- humor
The Pathology Blues
 Ed's Pathology Review for USMLE I
Ed's Pathology Review for USMLE I
EXAMINING THE LIVING CARDIOVASCULAR SYSTEM
Correlations with Pathology
Ed Friedlander MD "the pathology guy"
A group of you asked, in lieu of continuing into "lung", to integrate pathology
and the changes you have learned about on physical exam. Pathologists are the
big integrators and "why"-folks in most medical schools, so here goes....
0. Turn off the TV 1. Inspect 2. Palpate 3. Percuss 4. Auscultate
Valsalva: Reduces, then increases, preload
Leg lift: Increases preload
Handgrip: Increases afterload
Inspection! Apex impulse. Where? How big? Any others? Dextrocardia / situs
inversus. Cyanosis? Jugular venous distention? Anything else?
Jugular venous pulse peaks demystified....
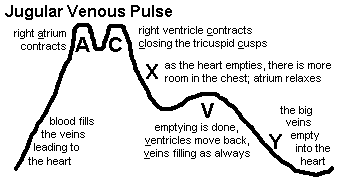
Big "A"'s: think of high pulmonary vascular resistance ("pulmonary
hypertension", why?
Big "V"'s: Tricuspid regurgitation (why)?
Estimating CVP: Kussmaul's sign in the neck veins in tamponade (why?)
Palpation! Right ("sternal") lift or heave. Big apex beat is "left lift" or
"left heave". Thrill -- palpable murmur. Feel PMI and carotids
simultaneously. Position of PMI can be measured; duration has most to do with
actual hypertrophy.
Percussion!: Bring your sharpie marker. Don't expect this to tell you as much
or as well as a chest x-ray.
Auscultation!: Bell (S3 and S4) vs. diaphragm (everything else). "I can't tell
you, professor, I am listening to diastole!" Inching along. Sitback and put
your elbows on your knees. Roll over on your left side and lean back against
me.
S1. What is it? Can anybody hear T1?
S2. What is it? P2 is delayed a bit during inspiration. Learning to tell if
a sound is split. Exaggerated split (i.e., P2 is always a little too late):
increased pulmonary vascular resistance (why?), left-to-right shunt (why?),
right bundle branch block (why?) Paradoxical split (i.e., A2 is always a
little too late and/or P2 a bit early); left bundle branch block (why?), some
normal folks. Loud A2: systemic hypertension. Loud P2: Pulmonary
hypertension.
S3: Waves-in-the-wall, the heart as kettledrum? "Tennessee". When do
you hear it? Why?
S4: Atrial sound. "Kentucky". When do you hear it? Why?
Ejection click: Little wiggles in the aortic and/or pulmonary valve leaflets as
they open.
Opening snap: Litle wiggles in the stiff leaflets of a tight mitral valve.
Midsystolic click: Barlow posterior leaflet being pulled tense, less when you
increase preload.
Murmurs mean turbulence. Volume of sound is proportionate to force and volume
of turbulent flow.
Systolic murmurs:
 Pectus
Pectus
Virtual Hospital

Pathological Chess

Taser Video
83.4 MB
7:26 min